当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Conformational Ensemble of Solvated LiO2 Determines the Growth of Li2O2 Crystals in Li–Air Batteries
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-07-27 , DOI: 10.1021/acs.jpcc.3c03004
Hongjiao Li 1, 2 , Tobias Schlöder 2 , Wolfgang Wenzel 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-07-27 , DOI: 10.1021/acs.jpcc.3c03004
Hongjiao Li 1, 2 , Tobias Schlöder 2 , Wolfgang Wenzel 2
Affiliation
|
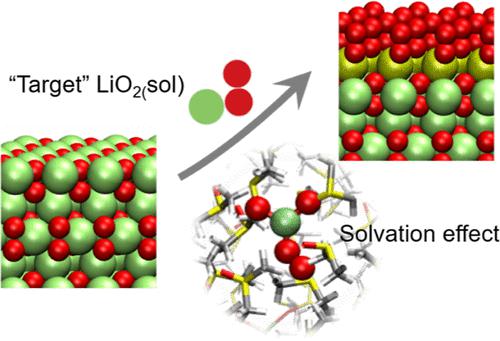
The high energy capacity of Li–air batteries with aprotic solvents in the electrolyte is directly associated with the formation of micrometer-sized Li2O2 crystals at the cathode. The growth process of the Li2O2 crystals during discharge is believed to proceed via a solution-mediated reaction pathway. Presently, the lack of understanding of the molecular processes that contribute to this pathway both in solution and on the surface limits the capacity to optimize electrolytes and electrodes for Li–air batteries. In this work, we examine the growth of Li2O2 crystals via the solution-mediated pathway in three common solvents with different nucleophilicities by ab initio simulations. A thermodynamic analysis indicates that the crystal growth mostly takes place on oxygen-terminated Li2O2 (0001) in a layer-by-layer fashion, which in turn proceeds best via a specific deformed conformation of LiO2 with a single Li–O bond [named as “target” LiO2(sol)] that is able to attach to defect sites on Li2O2(0001) and become a part of the crystal. Ab initio molecular dynamics simulations in three typical solvents [propylene carbonate (PC), dimethoxyethane (DME), and dimethyl sulfoxide (DMSO)] show that the interactions between the solvents and LiO2 affect the balance of the conformational populations of solvated LiO2, in the order of DMSO > DME > PC. This chain of arguments explains why solvents with a stronger solvating ability (i.e., a higher Gutmann donor number for aprotic solvents) are more favorable for the growth of Li2O2 crystals via the solution-mediated pathway and suggests that an electrolyte system with a high concentration of “target” LiO2(sol) would be ideal for the formation of large Li2O2 crystals and thereby high-capacity Li–air batteries.
中文翻译:

溶剂化 LiO2 的分子构象集合决定了 Li-空气电池中 Li2O2 晶体的生长
电解质中含有非质子溶剂的锂空气电池的高能量容量与阴极处微米尺寸的Li 2 O 2晶体的形成直接相关。放电期间Li 2 O 2晶体的生长过程被认为是通过溶液介导的反应途径进行的。目前,对溶液和表面上促进这一途径的分子过程缺乏了解,限制了优化锂空气电池电解质和电极的能力。在这项工作中,我们研究了 Li 2 O 2的生长通过从头模拟,在具有不同亲核性的三种常见溶剂中通过溶液介导的途径形成晶体。热力学分析表明,晶体生长主要发生在氧封端的 Li 2 O 2 (0001) 上,以逐层的方式进行,而这又通过具有单个 Li-O的 LiO 2的特定变形构象进行得最好。键[命名为“目标”LiO 2 (sol)],能够附着到Li 2 O 2 (0001) 上的缺陷位点并成为晶体的一部分。三种典型溶剂 [碳酸丙烯酯 (PC)、二甲氧基乙烷 (DME) 和二甲基亚砜 (DMSO)] 中的从头算分子动力学模拟表明,溶剂与 Li2O 之间的相互作用2对溶剂化LiO 2构象群平衡的影响顺序为DMSO>DME>PC。这一系列的论点解释了为什么具有较强溶剂化能力的溶剂(即,非质子溶剂的古特曼供体数较高)更有利于通过溶液介导途径生长 Li 2 O 2晶体,并表明具有高浓度的“目标”LiO 2 (sol) 对于形成大的 Li 2 O 2晶体以及由此形成高容量的锂空气电池来说是理想的。
更新日期:2023-07-27
中文翻译:

溶剂化 LiO2 的分子构象集合决定了 Li-空气电池中 Li2O2 晶体的生长
电解质中含有非质子溶剂的锂空气电池的高能量容量与阴极处微米尺寸的Li 2 O 2晶体的形成直接相关。放电期间Li 2 O 2晶体的生长过程被认为是通过溶液介导的反应途径进行的。目前,对溶液和表面上促进这一途径的分子过程缺乏了解,限制了优化锂空气电池电解质和电极的能力。在这项工作中,我们研究了 Li 2 O 2的生长通过从头模拟,在具有不同亲核性的三种常见溶剂中通过溶液介导的途径形成晶体。热力学分析表明,晶体生长主要发生在氧封端的 Li 2 O 2 (0001) 上,以逐层的方式进行,而这又通过具有单个 Li-O的 LiO 2的特定变形构象进行得最好。键[命名为“目标”LiO 2 (sol)],能够附着到Li 2 O 2 (0001) 上的缺陷位点并成为晶体的一部分。三种典型溶剂 [碳酸丙烯酯 (PC)、二甲氧基乙烷 (DME) 和二甲基亚砜 (DMSO)] 中的从头算分子动力学模拟表明,溶剂与 Li2O 之间的相互作用2对溶剂化LiO 2构象群平衡的影响顺序为DMSO>DME>PC。这一系列的论点解释了为什么具有较强溶剂化能力的溶剂(即,非质子溶剂的古特曼供体数较高)更有利于通过溶液介导途径生长 Li 2 O 2晶体,并表明具有高浓度的“目标”LiO 2 (sol) 对于形成大的 Li 2 O 2晶体以及由此形成高容量的锂空气电池来说是理想的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号