当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Perovskite Nanocrystals for Asymmetric Reactions: A Highly Enantioselective Strategy for Photocatalytic Synthesis of N–C Axially Chiral Heterocycles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-27 , DOI: 10.1021/jacs.3c04593 Kanchan Mishra 1 , Dylana Guyon 1 , Jovan San Martin 1 , Yong Yan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-27 , DOI: 10.1021/jacs.3c04593 Kanchan Mishra 1 , Dylana Guyon 1 , Jovan San Martin 1 , Yong Yan 1
Affiliation
|
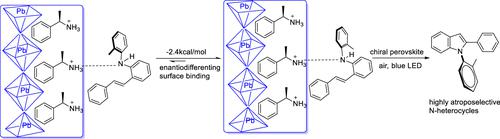
Catalytic approaches to generate enantiospecific chiral centers are the major premise of modern organic chemistry. Heterogeneous catalysis is responsible for the vast majority of chemical transformations, yet the direct employment of chiral solid catalysts for asymmetric synthesis is mostly overlooked. Here, we demonstrated that a heterogeneous metal-halide perovskite nanocrystal (NC) catalyst is active for asymmetric organic synthesis under visible-light activation. Chiral 1-phenylethylamine (PEA)-hybridized perovskite PEA/CsPbBr3 NC photocatalysts exhibit an enantioselective (up to 99% enantiomer excess, ee) avenue to produce N–C axially chiral N-heterocycles, i.e., N-arylindoles from N-arylamine photo-oxidation. Mechanistic investigation indicated a discriminated prochiral binding of the N-arylamine substrates onto the chiral-NC surface with ca. −2.4 kcal/mol enantiodifferentiation. Our perovskite NC heterogeneous catalytic system not only demonstrates a promising strategy to address the long-term challenges in atroposelective pharmaceutical scaffold synthesis but also paves the road to directly employ chiral solids for asymmetric synthesis.
中文翻译:

用于不对称反应的手性钙钛矿纳米晶:光催化合成 N-C 轴向手性杂环的高度对映选择性策略
产生对映特异性手性中心的催化方法是现代有机化学的主要前提。绝大多数化学转化是由多相催化引起的,但直接使用手性固体催化剂进行不对称合成却大多被忽视。在这里,我们证明了非均相金属卤化物钙钛矿纳米晶体(NC)催化剂在可见光活化下具有用于不对称有机合成的活性。手性 1-苯乙胺 (PEA) 杂化钙钛矿 PEA/CsPbBr 3 NC 光催化剂表现出对映选择性(对映异构体过量高达 99%,ee),可产生 N-C 轴向手性 N-杂环,即从N-芳基胺生成N-芳基吲哚光氧化。机理研究表明, N-芳基胺底物在手性-NC 表面上有明显的前手性结合,其结合力大约为 100 nm。 -2.4 kcal/mol 对映体分化。我们的钙钛矿NC多相催化系统不仅展示了解决肌体选择性药物支架合成中长期挑战的有前景的策略,而且为直接使用手性固体进行不对称合成铺平了道路。
更新日期:2023-07-27
中文翻译:

用于不对称反应的手性钙钛矿纳米晶:光催化合成 N-C 轴向手性杂环的高度对映选择性策略
产生对映特异性手性中心的催化方法是现代有机化学的主要前提。绝大多数化学转化是由多相催化引起的,但直接使用手性固体催化剂进行不对称合成却大多被忽视。在这里,我们证明了非均相金属卤化物钙钛矿纳米晶体(NC)催化剂在可见光活化下具有用于不对称有机合成的活性。手性 1-苯乙胺 (PEA) 杂化钙钛矿 PEA/CsPbBr 3 NC 光催化剂表现出对映选择性(对映异构体过量高达 99%,ee),可产生 N-C 轴向手性 N-杂环,即从N-芳基胺生成N-芳基吲哚光氧化。机理研究表明, N-芳基胺底物在手性-NC 表面上有明显的前手性结合,其结合力大约为 100 nm。 -2.4 kcal/mol 对映体分化。我们的钙钛矿NC多相催化系统不仅展示了解决肌体选择性药物支架合成中长期挑战的有前景的策略,而且为直接使用手性固体进行不对称合成铺平了道路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号