Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-07-26 , DOI: 10.1016/j.actbio.2023.07.038
Sydney Song 1 , Lindsey N Druschel 1 , E Ricky Chan 2 , Jeffrey R Capadona 1
|
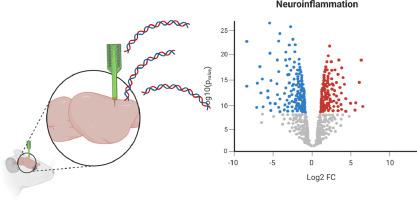
Brain-Machine Interface systems (BMIs) are clinically valuable devices that can provide functional restoration for patients with spinal cord injury or improved integration for patients requiring prostheses. Intracortical microelectrodes can record neuronal action potentials at a resolution necessary for precisely controlling BMIs. However, intracortical microelectrodes have a demonstrated history of progressive decline in the recording performance with time, inhibiting their usefulness. One major contributor to decreased performance is the neuroinflammatory response to the implanted microelectrodes. The neuroinflammatory response can lead to neurodegeneration and the formation of a glial scar at the implant site. Historically, histological imaging of relatively few known cellular and protein markers has characterized the neuroinflammatory response to implanted microelectrode arrays. However, neuroinflammation requires many molecular players to coordinate the response - meaning traditional methods could result in an incomplete understanding. Taking advantage of recent advancements in tools to characterize the relative or absolute DNA/RNA expression levels, a few groups have begun to explore gene expression at the microelectrode-tissue interface. We have utilized a custom panel of ∼813 neuroinflammatory-specific genes developed with NanoString for bulk tissue analysis at the microelectrode-tissue interface. Our previous studies characterized the acute innate immune response to intracortical microelectrodes. Here we investigated the gene expression at the microelectrode-tissue interface in wild-type (WT) mice chronically implanted with nonfunctioning probes. We found 28 differentially expressed genes at chronic time points (4WK, 8WK, and 16WK), many in the complement and extracellular matrix system. Further, the expression levels were relatively stable over time. Genes identified here represent chronic molecular players at the microelectrode implant sites and potential therapeutic targets for the long-term integration of microelectrodes.
Statement of significance
Intracortical microelectrodes can record neuronal action potentials at a resolution necessary for the precise control of Brain-Machine Interface systems (BMIs). However, intracortical microelectrodes have a demonstrated history of progressive declines in the recording performance with time, inhibiting their usefulness. One major contributor to the decline in these devices is the neuroinflammatory response against the implanted microelectrodes. Historically, neuroinflammation to implanted microelectrode arrays has been characterized by histological imaging of relatively few known cellular and protein markers. Few studies have begun to develop a more in-depth understanding of the molecular pathways facilitating device-mediated neuroinflammation. Here, we are among the first to identify genetic pathways that could represent targets to improve the host response to intracortical microelectrodes, and ultimately device performance.
中文翻译:

参与皮质内微电极慢性反应的基因的差异表达
脑机接口系统(BMI)是具有临床价值的设备,可以为脊髓损伤患者提供功能恢复,或为需要假肢的患者提供改善的整合能力。皮质内微电极可以以精确控制体重指数所需的分辨率记录神经元动作电位。然而,皮质内微电极的记录性能随着时间的推移而逐渐下降,从而抑制了它们的实用性。导致性能下降的主要原因之一是对植入微电极的神经炎症反应。神经炎症反应可导致神经变性并在植入部位形成神经胶质疤痕。从历史上看,相对较少的已知细胞和蛋白质标记物的组织学成像已经表征了对植入微电极阵列的神经炎症反应。然而,神经炎症需要许多分子参与者来协调反应——这意味着传统方法可能会导致理解不完整。利用表征相对或绝对 DNA/RNA 表达水平的工具的最新进展,一些研究小组已经开始探索微电极-组织界面的基因表达。我们利用 NanoString 开发的约 813 个神经炎症特异性基因的定制面板,在微电极-组织界面进行大量组织分析。我们之前的研究描述了对皮质内微电极的急性先天免疫反应。在这里,我们研究了长期植入无功能探针的野生型(WT)小鼠微电极-组织界面的基因表达。 我们发现了 28 个在慢性时间点(4WK、8WK 和 16WK)差异表达的基因,其中许多位于补体和细胞外基质系统中。此外,随着时间的推移,表达水平相对稳定。这里确定的基因代表微电极植入部位的慢性分子参与者和微电极长期整合的潜在治疗靶点。
重要性声明
皮质内微电极可以以精确控制脑机接口系统(BMI)所需的分辨率记录神经元动作电位。然而,皮质内微电极的记录性能随着时间的推移而逐渐下降,从而抑制了它们的实用性。这些设备衰落的主要原因之一是针对植入微电极的神经炎症反应。历史上,植入微电极阵列的神经炎症通过相对较少的已知细胞和蛋白质标记物的组织学成像来表征。很少有研究开始对促进装置介导的神经炎症的分子途径进行更深入的了解。在这里,我们是第一批确定可以代表改善宿主对皮质内微电极反应并最终提高设备性能的目标的遗传途径的人之一。







































 京公网安备 11010802027423号
京公网安备 11010802027423号