European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-07-26 , DOI: 10.1016/j.ejmech.2023.115674 Katerina Novotna 1 , Ajit G Thomas 2 , Ondrej Stepanek 3 , Brennan Murphy 3 , Niyada Hin 2 , Jan Skacel 2 , Louis Mueller 3 , Lukas Tenora 4 , Arindom Pal 3 , Jesse Alt 2 , Ying Wu 2 , James Paule 2 , Rana Rais 5 , Barbara S Slusher 5 , Takashi Tsukamoto 5
|
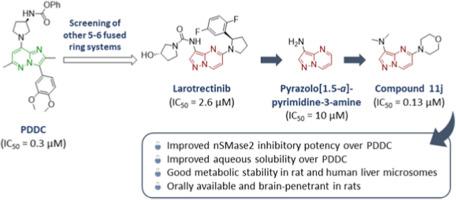
Neutral sphingomyelinase 2 (nSMase2) has gained increasing attention as a therapeutic target to regulate ceramide production in various disease conditions. Phenyl (R)-(1-(3-(3,4-dimethoxyphenyl)-2,6-dimethylimidazo[1,2-b]pyridazin-8-yl)-pyrrolidin-3-yl)carbamate (PDDC) is a submicromolar nSMase2 inhibitor and has been widely used to study the pharmacological effects of nSMase2 inhibition. Through screening of compounds containing a bicyclic 5–6 fused ring, larotrectinib containing a pyrazolo[1,5-a]pyrimidine ring was identified as a low micromolar inhibitor of nSMase2. This prompted us to investigate the pyrazolo[1,5-a]pyrimidin-3-amine ring as a novel scaffold to replace the imidazo[1,2-b]pyridazine-8-amine ring of PDDC. A series of molecules containing a pyrazolo[1,5-a]pyrimidin-3-amine ring were synthesized and tested for their ability to inhibit human nSMase2. Several compounds exhibited nSMase2 inhibitory potency superior to that of PDDC. Among these, N,N-dimethyl-5-morpholinopyrazolo[1,5-a]pyrimidin-3-amine (11j) was found to be metabolically stable in liver microsomes and orally available with a favorable brain-to-plasma ratio, demonstrating the potential of pyrazolo[1,5-a]pyrimidine ring as an effective scaffold for nSMase2 inhibition.
中文翻译:

基于吡唑并[1,5-a]嘧啶-3-胺支架的中性鞘磷脂酶 2 抑制剂
中性鞘磷脂酶 2 (nSMase2) 作为调节各种疾病中神经酰胺产生的治疗靶点而受到越来越多的关注。苯基 (R)-(1-(3-(3,4-二甲氧基苯基)-2,6-二甲基咪唑并[1,2-b]吡咪嗪-8-基)-吡咯烷-3-基)氨基甲酸酯 (PDDC) 是一种亚微摩尔 nSMase2 抑制剂,已被广泛用于研究 nSMase2 抑制的药理作用。通过筛选含有双环 5-6 稠合环的化合物,含有吡唑并[1,5-a] 嘧啶环的 larotrectinib 被鉴定为 nSMase2 的低微摩尔抑制剂。这促使我们研究吡唑并[1,5-a]嘧啶-3-胺环作为替代PDDC的咪唑[1,2-b]吡吒嗪-8-胺环的新型支架。合成了一系列含有吡唑并[1,5-a]嘧啶-3-胺环的分子,并测试了它们抑制人 nSMase2 的能力。几种化合物表现出优于 PDDC 的 nSMase2 抑制效力。其中,发现 N,N-二甲基-5-吗啉代吡唑并[1,5-a]嘧啶-3-胺 (11j) 在肝微粒体中代谢稳定,并且以良好的脑与血浆比口服获得,证明了吡唑并[1,5-a]嘧啶环作为 nSMase2 抑制的有效支架的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号