当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and structure determination of a synthetic cannabinoid CUMYL-THPINACA metabolite with differentiation between the ortho-, meta-, and para-hydroxyl positions of the cumyl moiety
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2023-07-24 , DOI: 10.1002/dta.3548 Yuki Azuma 1 , Takahiro Doi 1 , Akiko Asada 1 , Misa Tanaka 1 , Takaomi Tagami 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2023-07-24 , DOI: 10.1002/dta.3548 Yuki Azuma 1 , Takahiro Doi 1 , Akiko Asada 1 , Misa Tanaka 1 , Takaomi Tagami 1
Affiliation
|
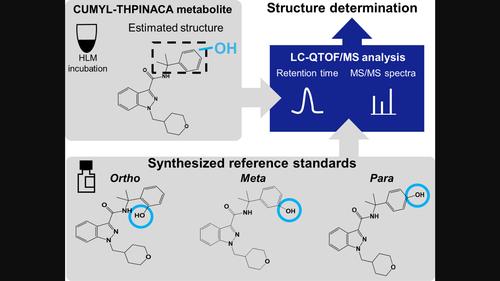
Synthetic cannabinoids, a type of new psychoactive substances, are likely to be rapidly metabolized; thus, the detection of their metabolites, rather than the parent compound, is a common method used to prove drug consumption. Although the analysis of metabolites is generally performed by mass spectrometry, it is limited to structural estimation because of few commercially available standards. In particular, distinguishing between positional isomers is difficult. Synthetic cannabinoids with a cumyl moiety can be hydroxylated at the cumyl moiety during metabolism, but it remains unclear whether the hydroxylation occurs at the ortho, meta, or para position. This study determined the structures of a metabolite formed by mono-hydroxylation at the cumyl moiety of the synthetic cannabinoid CUMYL-THPINACA, used as a model compound. Chemical synthesis was performed to create possible metabolites with one hydroxyl group at the ortho, meta, or para positions of the cumyl moiety. Using the synthesized metabolites and liquid chromatography-quadrupole time-of-flight mass spectrometry, the metabolite detected in the microsomal reaction of CUMYL-THPINACA was identified as a compound mono-hydroxylated at the para position based on retention time and product ion spectra. Moreover, the rapid metabolism of CUMYL-THPINACA was demonstrated with an in vitro half-life of 4.9 min and the identified metabolite could be detected for a relatively long time in vitro. The synthesized metabolite may be utilized as a good reference standard for proof of CUMYL-THPINACA consumption. These findings have potential applications in the synthesis of metabolites of other synthetic cannabinoids bearing a cumyl moiety.
中文翻译:

合成大麻素 CUMYL-THPINACA 代谢物的合成和结构测定,并区分枯基部分的邻位、间位和对位羟基位置
合成大麻素是一种新型精神活性物质,可能会被快速代谢;因此,检测其代谢物而不是母体化合物是证明药物消耗的常用方法。尽管代谢物的分析通常通过质谱法进行,但由于商业上可用的标准很少,因此仅限于结构估计。特别是,区分位置异构体很困难。具有枯基部分的合成大麻素在代谢过程中可以在枯基部分羟基化,但目前尚不清楚羟基化是否发生在邻位、间位或对位。本研究确定了合成大麻素 CUMYL-THPINACA 枯基部分单羟基化形成的代谢物的结构,用作模型化合物。进行化学合成以产生在枯基部分的邻位、间位或对位具有一个羟基的可能代谢物。利用合成的代谢物和液相色谱-四极杆飞行时间质谱,根据保留时间和产物离子谱,将 CUMYL-THPINACA 微粒体反应中检测到的代谢物鉴定为在对位单羟基化的化合物。此外,CUMYL-THPINACA 的快速代谢被证明,体外半衰期为 4.9 分钟,并且可以在体外相对较长的时间内检测到所鉴定的代谢物。合成的代谢物可用作 CUMYL-THPINACA 消耗证明的良好参考标准。这些发现在合成带有枯基部分的其他合成大麻素的代谢物方面具有潜在的应用。
更新日期:2023-07-24
中文翻译:

合成大麻素 CUMYL-THPINACA 代谢物的合成和结构测定,并区分枯基部分的邻位、间位和对位羟基位置
合成大麻素是一种新型精神活性物质,可能会被快速代谢;因此,检测其代谢物而不是母体化合物是证明药物消耗的常用方法。尽管代谢物的分析通常通过质谱法进行,但由于商业上可用的标准很少,因此仅限于结构估计。特别是,区分位置异构体很困难。具有枯基部分的合成大麻素在代谢过程中可以在枯基部分羟基化,但目前尚不清楚羟基化是否发生在邻位、间位或对位。本研究确定了合成大麻素 CUMYL-THPINACA 枯基部分单羟基化形成的代谢物的结构,用作模型化合物。进行化学合成以产生在枯基部分的邻位、间位或对位具有一个羟基的可能代谢物。利用合成的代谢物和液相色谱-四极杆飞行时间质谱,根据保留时间和产物离子谱,将 CUMYL-THPINACA 微粒体反应中检测到的代谢物鉴定为在对位单羟基化的化合物。此外,CUMYL-THPINACA 的快速代谢被证明,体外半衰期为 4.9 分钟,并且可以在体外相对较长的时间内检测到所鉴定的代谢物。合成的代谢物可用作 CUMYL-THPINACA 消耗证明的良好参考标准。这些发现在合成带有枯基部分的其他合成大麻素的代谢物方面具有潜在的应用。








































 京公网安备 11010802027423号
京公网安备 11010802027423号