当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia Decomposition over Water-Durable Hexagonal BaTiO3−xNy-Supported Ni Catalysts
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2023-07-25 , DOI: 10.1002/aenm.202301286 Kiya Ogasawara 1 , Masayoshi Miyazaki 1 , Kazuki Miyashita 1 , Hitoshi Abe 2 , Yasuhiro Niwa 2 , Masato Sasase 1 , Masaaki Kitano 1, 3 , Hideo Hosono 1, 4
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2023-07-25 , DOI: 10.1002/aenm.202301286 Kiya Ogasawara 1 , Masayoshi Miyazaki 1 , Kazuki Miyashita 1 , Hitoshi Abe 2 , Yasuhiro Niwa 2 , Masato Sasase 1 , Masaaki Kitano 1, 3 , Hideo Hosono 1, 4
Affiliation
|
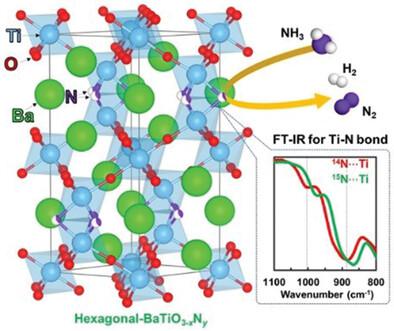
Nickel is a promising candidate as an alternative to ruthenium for an ammonia decomposition catalyst. However, the performance of Ni-based catalysts for ammonia decomposition is still not sufficient to achieve a good hydrogen production rate under low-temperature because the weak nitrogen affinity of Ni reduces the frequency of the ammonia decomposition reaction. Here, it is reported that Ni supported on barium titanium oxynitride (Ni/h-BaTiO3−xNy) with a hexagonal structure acts as a highly active and water-durable catalyst for ammonia decomposition. The operation temperature is reduced by over 140 °C when N3− ions are substituted onto the O2− sites of the BaTiO3 lattice, and the Ni/h-BaTiO3−xNy catalyst significantly outperforms conventional oxide-supported Ni catalysts for ammonia decomposition. Furthermore, the activity of Ni/h-BaTiO3−xNy remains unchanged after exposure to water. The 15NH3 decomposition reaction and Fourier transform-infrared spectroscopy (FT-IR) measurements reveal that lattice nitrogen vacancy sites on h-BaTiO3−xNy function as the active sites for ammonia decomposition. The ammonia decomposition activity of Ni/h-BaTiO3−xNy is also higher than that of the Ni/h-BaTiO3−xHy oxyhydride catalyst, making a contrast to the activity trend in ammonia synthesis.
中文翻译:

耐水六方 BaTiO3−xNy 负载 Ni 催化剂上的氨分解
镍是作为氨分解催化剂的钌替代品的有前途的候选者。然而,镍基氨分解催化剂的性能仍然不足以在低温下实现良好的产氢速率,因为镍的弱氮亲和力降低了氨分解反应的频率。据报道,负载在具有六方结构的氮氧化钡钛(Ni/ h -BaTiO 3− x N y)上的Ni可作为氨分解的高活性和耐水性催化剂。当 N 3−离子取代BaTiO 3的 O 2−位点时,工作温度降低了 140 °C 以上Ni/ h -BaTiO 3− x N y催化剂在氨分解方面显着优于传统的氧化物负载 Ni 催化剂。此外,Ni/ h -BaTiO 3− x N y的活性在暴露于水后保持不变。15 NH 3分解反应和傅里叶变换红外光谱(FT-IR)测量表明h -BaTiO 3− x N y上的晶格氮空位位作为氨分解的活性位点。Ni/ h -BaTiO的氨分解活性3− x N y也高于 Ni/ h -BaTiO 3− x H y氢氧化物催化剂,与氨合成中的活性趋势形成对比。
更新日期:2023-07-25
中文翻译:

耐水六方 BaTiO3−xNy 负载 Ni 催化剂上的氨分解
镍是作为氨分解催化剂的钌替代品的有前途的候选者。然而,镍基氨分解催化剂的性能仍然不足以在低温下实现良好的产氢速率,因为镍的弱氮亲和力降低了氨分解反应的频率。据报道,负载在具有六方结构的氮氧化钡钛(Ni/ h -BaTiO 3− x N y)上的Ni可作为氨分解的高活性和耐水性催化剂。当 N 3−离子取代BaTiO 3的 O 2−位点时,工作温度降低了 140 °C 以上Ni/ h -BaTiO 3− x N y催化剂在氨分解方面显着优于传统的氧化物负载 Ni 催化剂。此外,Ni/ h -BaTiO 3− x N y的活性在暴露于水后保持不变。15 NH 3分解反应和傅里叶变换红外光谱(FT-IR)测量表明h -BaTiO 3− x N y上的晶格氮空位位作为氨分解的活性位点。Ni/ h -BaTiO的氨分解活性3− x N y也高于 Ni/ h -BaTiO 3− x H y氢氧化物催化剂,与氨合成中的活性趋势形成对比。






























 京公网安备 11010802027423号
京公网安备 11010802027423号