Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reshaping Inner Helmholtz Layer and Electrolyte Structure via Multifunctional Organic Molecule Enabling Dendrite-Free Zn Metal Anode
Small ( IF 13.0 ) Pub Date : 2023-07-23 , DOI: 10.1002/smll.202304751 Chang Liu 1 , Wen-Bin Jiang 1 , Dan Xie 1 , Wan-Yue Diao 1 , Fang-Yu Tao 1 , Xin-Zhe Wang 1 , Hai-Zhu Sun 1 , Wen-Liang Li 1 , Xing-Long Wu 1, 2 , Jing-Ping Zhang 1
Small ( IF 13.0 ) Pub Date : 2023-07-23 , DOI: 10.1002/smll.202304751 Chang Liu 1 , Wen-Bin Jiang 1 , Dan Xie 1 , Wan-Yue Diao 1 , Fang-Yu Tao 1 , Xin-Zhe Wang 1 , Hai-Zhu Sun 1 , Wen-Liang Li 1 , Xing-Long Wu 1, 2 , Jing-Ping Zhang 1
Affiliation
|
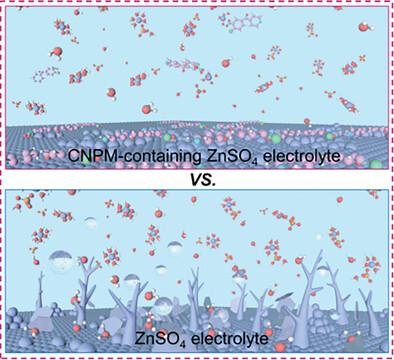
The dendrite growth and parasitic reactions that occur on Zn metal anode (ZMA)/electrolyte interface hinder the development of aqueous zinc ion batteries (AZIBs) in next-generation renewable energy storage systems. Fortunately, reconstructing the inner Helmholtz layer (IHL) by introducing an electrolyte additive, is viewed as one of the most promising strategies to harvest the stable ZMA. Herein, (4-chloro-3-nitrophenyl) (pyridin-4-yl) methanone (CNPM) with quadruple functional groups is introduced into the ZnSO4 electrolyte to reshape the interface between ZMA and electrolyte and change the solvation structure of Zn2+. Density functional theory (DFT) calculations manifest that the ─C═O, ─Cl, ─C═N─, and ─NO2 functional groups of CNPM interact with metallic Zn simultaneously and adsorb on the ZMA surface in a parallel arrangement manner, thus forming a water-poor IHL and creating well-arranged ion transportation channels. Furthermore, theoretical calculations and experimental results demonstrate that CNPM absorbed on the Zn anode surface can serve as zincophilic sites for inducing uniform Zn deposition along the (002) plane. Benefiting from the synergistic effect of these functions, the dendrite growth and parasitic reactions are suppressed significantly. As a result, ZMA exhibits a long cycle life (2900 h) and high coulombic efficiency (CE) (500 cycles) in the ZnSO4+CNPM electrolyte.
中文翻译:

通过多功能有机分子重塑内亥姆霍兹层和电解质结构,实现无枝晶的锌金属阳极
锌金属阳极(ZMA)/电解质界面上发生的枝晶生长和寄生反应阻碍了水性锌离子电池(AZIB)在下一代可再生能源存储系统中的发展。幸运的是,通过引入电解质添加剂来重建内亥姆霍兹层(IHL)被视为获得稳定 ZMA 的最有前途的策略之一。在此,将具有四官能团的(4-氯-3-硝基苯基)(吡啶-4-基)甲酮(CNPM)引入到ZnSO 4 电解质中,重塑ZMA和电解质之间的界面并改变Zn 2+的溶剂化结构。密度泛函理论(DFT)计算表明CNPM的─C=O、─Cl、─C=N─和─NO 2官能团同时与金属Zn相互作用并以平行排列的方式吸附在ZMA表面,从而形成缺水的国际人道法并创建布局良好的离子运输通道。此外,理论计算和实验结果表明,吸附在Zn阳极表面上的CNPM可以作为亲锌位点,诱导Zn沿(002)面均匀沉积。受益于这些功能的协同作用,枝晶生长和寄生反应被显着抑制。因此,ZMA在ZnSO 4 +CNPM电解质中表现出长循环寿命(2900小时)和高库仑效率(CE)(500次循环)。
更新日期:2023-07-23
中文翻译:

通过多功能有机分子重塑内亥姆霍兹层和电解质结构,实现无枝晶的锌金属阳极
锌金属阳极(ZMA)/电解质界面上发生的枝晶生长和寄生反应阻碍了水性锌离子电池(AZIB)在下一代可再生能源存储系统中的发展。幸运的是,通过引入电解质添加剂来重建内亥姆霍兹层(IHL)被视为获得稳定 ZMA 的最有前途的策略之一。在此,将具有四官能团的(4-氯-3-硝基苯基)(吡啶-4-基)甲酮(CNPM)引入到ZnSO 4 电解质中,重塑ZMA和电解质之间的界面并改变Zn 2+的溶剂化结构。密度泛函理论(DFT)计算表明CNPM的─C=O、─Cl、─C=N─和─NO 2官能团同时与金属Zn相互作用并以平行排列的方式吸附在ZMA表面,从而形成缺水的国际人道法并创建布局良好的离子运输通道。此外,理论计算和实验结果表明,吸附在Zn阳极表面上的CNPM可以作为亲锌位点,诱导Zn沿(002)面均匀沉积。受益于这些功能的协同作用,枝晶生长和寄生反应被显着抑制。因此,ZMA在ZnSO 4 +CNPM电解质中表现出长循环寿命(2900小时)和高库仑效率(CE)(500次循环)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号