当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of highly functional quinazolins via metal-free, visible-light-enabled radical cascade arylation/cyclization of fluorinated imidoyl isothiocyanates
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2023-07-23 , DOI: 10.1016/j.gresc.2023.07.003 Yan Liang , Weigen Du , Xiaoqiong Zeng , Tiebo Xiao , Yubo Jiang
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2023-07-23 , DOI: 10.1016/j.gresc.2023.07.003 Yan Liang , Weigen Du , Xiaoqiong Zeng , Tiebo Xiao , Yubo Jiang
|
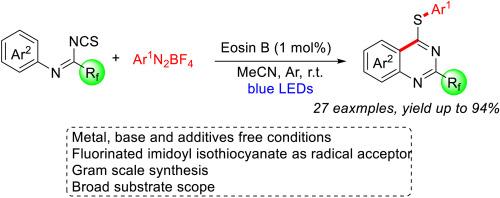
A metal-free, efficient and easy-to-hand protocol for the synthesis of 2-perfluoroalkylquinazolins has been achieved by Eosin B catalyzed radical cascade arylation/cyclization reaction of fluorinated imidoyl isothiocyanates with aryldiazonium Salts. A variety of highly functionalized quinozaline derivatives bearing pharmaceutically important thiol and fluoroalkyl groups were efficiently assembled with broad substrate scope and good functional group tolerance. A series of mechanism experiments indicate that this reaction undergoes a radical cascade arylation/cyclization pathway.
中文翻译:

通过氟化异硫氰酸咪酰亚胺酰亚胺酯的无金属、可见光自由基级联芳基化/环化合成高功能喹唑啉
通过曙红 B 催化氟化异硫氰酸咪酰亚胺酯与芳基叠氮盐的自由基级联芳基化/环化反应,实现了一种无金属、高效且易于操作的合成 2-全氟烷基喹唑啉的方案。各种具有重要药学意义的巯基和氟烷基的高功能化喹啉衍生物被有效地组装,具有广泛的底物范围和良好的官能团耐受性。一系列机理实验表明,该反应经历了自由基级联芳基化/环化途径。
更新日期:2023-07-23
中文翻译:

通过氟化异硫氰酸咪酰亚胺酰亚胺酯的无金属、可见光自由基级联芳基化/环化合成高功能喹唑啉
通过曙红 B 催化氟化异硫氰酸咪酰亚胺酯与芳基叠氮盐的自由基级联芳基化/环化反应,实现了一种无金属、高效且易于操作的合成 2-全氟烷基喹唑啉的方案。各种具有重要药学意义的巯基和氟烷基的高功能化喹啉衍生物被有效地组装,具有广泛的底物范围和良好的官能团耐受性。一系列机理实验表明,该反应经历了自由基级联芳基化/环化途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号