Molecular Therapy ( IF 12.1 ) Pub Date : 2023-07-22 , DOI: 10.1016/j.ymthe.2023.07.013 Samantha Powers 1 , Shibi Likhite 2 , Kamal K Gadalla 3 , Carlos J Miranda 2 , Amy J Huffenberger 2 , Cassandra Dennys 2 , Kevin D Foust 4 , Pablo Morales 5 , Christopher R Pierson 6 , Federica Rinaldi 2 , Stephanie Perry 2 , Brad Bolon 7 , Nicolas Wein 8 , Stuart Cobb 3 , Brian K Kaspar 8 , Kathrin C Meyer 9
|
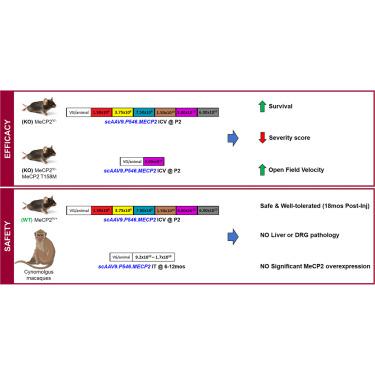
The AAV9 gene therapy vector presented in this study is safe in mice and non-human primates and highly efficacious without causing overexpression toxicity, a major challenge for clinical translation of Rett syndrome gene therapy vectors to date. Our team designed a new truncated methyl-CpG-binding protein 2 (MECP2) promoter allowing widespread expression of MECP2 in mice and non-human primates after a single injection into the cerebrospinal fluid without causing overexpression symptoms up to 18 months after injection. Additionally, this new vector is highly efficacious at lower doses compared with previous constructs as demonstrated in extensive efficacy studies performed by two independent laboratories in two different Rett syndrome mouse models carrying either a knockout or one of the most frequent human mutations of Mecp2. Overall, data from this multicenter study highlight the efficacy and safety of this gene therapy construct, making it a promising candidate for first-in-human studies to treat Rett syndrome.
中文翻译:

新型 MECP2 基因疗法在使用两种 Rett 综合征小鼠模型的多中心研究中有效,并且在非人类灵长类动物中是安全的
本研究中提出的 AAV9 基因治疗载体在小鼠和非人类灵长类动物中是安全的,并且非常有效,不会引起过度表达毒性,这是迄今为止雷特综合征基因治疗载体临床转化的主要挑战。我们的团队设计了一种新的截短的甲基 CpG 结合蛋白 2 (MECP2) 启动子,使 MECP2 在单次注射到脑脊液后在小鼠和非人类灵长类动物中广泛表达,而在注射后 18 个月内不会引起过度表达症状。此外,与之前的构建体相比,这种新载体在较低剂量下也非常有效,两个独立实验室在两种不同的雷特综合征小鼠模型中进行的广泛功效研究证明了这一点,这些小鼠模型携带 Mecp2 敲除或最常见的人类突变之一。总体而言,这项多中心研究的数据强调了这种基因治疗结构的有效性和安全性,使其成为治疗雷特综合征的首次人体研究的有希望的候选者。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号