当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of G12 Mutations on the Structure of K-Ras Probed by Ultraviolet Photodissociation Mass Spectrometry
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-10-03 , DOI: 10.1021/jacs.6b04474
Michael B. Cammarata 1 , Christopher L. Schardon 1 , M. Rachel Mehaffey 1 , Jake Rosenberg 1 , Jonathan Singleton 1 , Walter Fast 1 , Jennifer S. Brodbelt 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-10-03 , DOI: 10.1021/jacs.6b04474
Michael B. Cammarata 1 , Christopher L. Schardon 1 , M. Rachel Mehaffey 1 , Jake Rosenberg 1 , Jonathan Singleton 1 , Walter Fast 1 , Jennifer S. Brodbelt 1
Affiliation
|
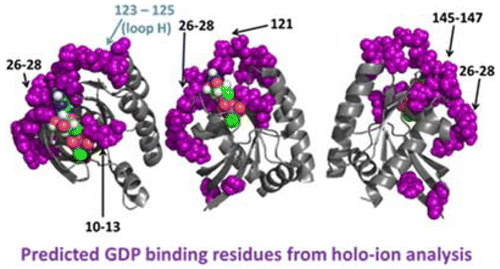
Single-residue mutations at Gly12 (G12X) in the GTP-ase protein K-Ras can lead to activation of different downstream signaling pathways, depending on the identity of the mutation, through a poorly defined mechanism. Herein, native mass spectrometry combined with top-down ultraviolet photodissociation (UVPD) was employed to investigate the structural changes occurring from G12X mutations of K-Ras. Complexes between K-Ras or the G12X mutants and guanosine 5'-diphosphate (GDP) or GDPnP (a stable GTP analogue) were transferred to the gas phase by nano-electrospray ionization and characterized using UVPD. Variations in the efficiencies of backbone cleavages were observed upon substitution of GDPnP for GDP as well as for the G12X mutants relative to wild-type K-Ras. An increase in the fragmentation efficiency in the segment containing the first 50 residues was observed for the K-Ras/GDPnP complexes relative to the K-Ras/GDP complexes, whereas a decrease in fragmentation efficiency occurred in the segment containing the last 100 residues. Within these general regions, the specific residues at which changes in fragmentation efficiency occurred correspond to the phosphate and guanine binding regions, respectively, and are indicative of a change in the binding motif upon replacement of the ligand (GDP versus GDPnP). Notably, unique changes in UVPD were observed for each G12X mutant with the cysteine and serine mutations exhibiting similar UVPD changes whereas the valine mutation was significantly different. These findings suggest a mechanism that links the identity of the G12X substitution to different downstream effects through long-range conformational or dynamic effects as detected by variations in UVPD fragmentation.
中文翻译:

G12 突变对紫外光解质谱法探测的 K-Ras 结构的影响
GTP-ase 蛋白 K-Ras 中 Gly12 (G12X) 的单残基突变可通过定义不明确的机制导致不同下游信号通路的激活,具体取决于突变的身份。在此,本机质谱结合自上而下的紫外光解离 (UVPD) 被用来研究 K-Ras 的 G12X 突变引起的结构变化。K-Ras 或 G12X 突变体与鸟苷 5'-二磷酸 (GDP) 或 GDPnP(稳定的 GTP 类似物)之间的复合物通过纳米电喷雾电离转移到气相,并使用 UVPD 进行表征。在 GDPnP 替代 GDP 以及 G12X 突变体相对于野生型 K-Ras 时,观察到骨架切割效率的变化。相对于 K-Ras/GDP 复合物,观察到 K-Ras/GDPnP 复合物在含有前 50 个残基的片段中的片段化效率增加,而在含有最后 100 个残基的片段中片段化效率下降。在这些一般区域内,发生片段化效率变化的特定残基分别对应于磷酸盐和鸟嘌呤结合区域,并表明配体替换后结合基序的变化(GDP 与 GDPnP)。值得注意的是,每个 G12X 突变体都观察到 UVPD 的独特变化,其中半胱氨酸和丝氨酸突变表现出类似的 UVPD 变化,而缬氨酸突变则显着不同。
更新日期:2016-10-03
中文翻译:

G12 突变对紫外光解质谱法探测的 K-Ras 结构的影响
GTP-ase 蛋白 K-Ras 中 Gly12 (G12X) 的单残基突变可通过定义不明确的机制导致不同下游信号通路的激活,具体取决于突变的身份。在此,本机质谱结合自上而下的紫外光解离 (UVPD) 被用来研究 K-Ras 的 G12X 突变引起的结构变化。K-Ras 或 G12X 突变体与鸟苷 5'-二磷酸 (GDP) 或 GDPnP(稳定的 GTP 类似物)之间的复合物通过纳米电喷雾电离转移到气相,并使用 UVPD 进行表征。在 GDPnP 替代 GDP 以及 G12X 突变体相对于野生型 K-Ras 时,观察到骨架切割效率的变化。相对于 K-Ras/GDP 复合物,观察到 K-Ras/GDPnP 复合物在含有前 50 个残基的片段中的片段化效率增加,而在含有最后 100 个残基的片段中片段化效率下降。在这些一般区域内,发生片段化效率变化的特定残基分别对应于磷酸盐和鸟嘌呤结合区域,并表明配体替换后结合基序的变化(GDP 与 GDPnP)。值得注意的是,每个 G12X 突变体都观察到 UVPD 的独特变化,其中半胱氨酸和丝氨酸突变表现出类似的 UVPD 变化,而缬氨酸突变则显着不同。

































 京公网安备 11010802027423号
京公网安备 11010802027423号