当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolation, Structure Elucidation and in Vitro Anticancer Activity of Phytochemical Constituents of Goniothalamus wynaadensis Bedd. and Identification of α-Tubulin as a Putative Molecular Target by in Silico Study
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-07-21 , DOI: 10.1002/cbdv.202300371 Akanksha Sharma 1 , Doddabasappa Talimarada 1 , Sundar N Dhuri 2 , Venkata Narayanan Naranammalpuram Sundaram 1 , Duraippandi Palanimutu 1 , Harish Holla 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-07-21 , DOI: 10.1002/cbdv.202300371 Akanksha Sharma 1 , Doddabasappa Talimarada 1 , Sundar N Dhuri 2 , Venkata Narayanan Naranammalpuram Sundaram 1 , Duraippandi Palanimutu 1 , Harish Holla 1
Affiliation
|
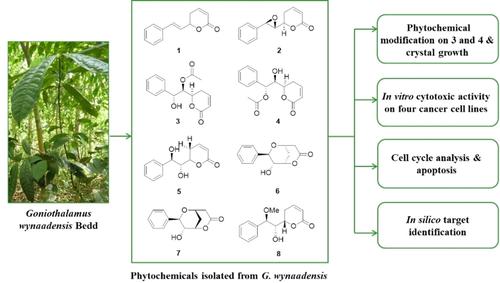
The phytochemical analysis of ethyl acetate and methanol extract of Goniothalamus wynaadensis Bedd. leaves led to an isolation of eight (1–8) known molecules, among them seven (2–8) isolated for the first time from this species, which includes (+)-goniothalamin oxide (2), goniodiol-7-monoacetate (3), goniodiol-8-monoacetate (4), goniodiol (5), (+)-8-epi-9-deoxygoniopypyrone (6) etc. The phytochemical modification by acetylation of 3 and 4 gave goniodiol diacetate (9) with absolute configuration (6R, 7R, 8R) confirmed by single crystal X-ray diffraction. Compounds 3–9 were cytotoxic against breast, ovarian, prostate and colon cancer cell lines with IC50<10 μM. Cell cycle analysis and Annexin-V assay on MDA-MB-231 cell using goniodiol-7-monoacetate (3) exhibited apoptotic response as well as necrotic response and showed cell proliferation arrest at G2/M phase. An in silico target identification for these molecules was carried out with an α-tubulin protein target by covalent docking. To gain an in-depth understanding and identify the stability of these protein-ligand complexes on thermodynamic energy levels, further assessment of the isolated molecules binding to the Cys-316 of α-tubulin was performed based on reaction energetic analysis via DFT studies which hinted the isolated molecules may be α-tubulin inhibitors similar to Pironetin. Molecular dynamics reiterated the observations.
中文翻译:

Goniothalamus wynaadensis Bedd 植物化学成分的分离、结构解析和体外抗癌活性。通过计算机模拟研究鉴定 α-微管蛋白作为假定的分子靶标
Goniothalamus wynaadensis Bedd的乙酸乙酯和甲醇提取物的植物化学分析。叶子导致分离出八 ( 1 – 8 ) 个已知分子,其中七 ( 2 – 8 ) 是首次从该物种中分离出来,其中包括 (+)-goniothalamin 氧化物 ( 2 )、goniodiol-7-monoacetate ( 3 )、gonidiol-8-monoacetate ( 4 )、goniodiol ( 5 )、(+)-8-epi-9-deoxygoniopypyrone ( 6 ) 等。通过乙酰化3和4进行植物化学修饰,得到 gonidiol diacetate ( 9 ),其绝对值通过单晶X射线衍射证实了构型(6R , 7R , 8R ) 。化合物3 – 9对乳腺癌、卵巢癌、前列腺癌和结肠癌细胞系具有细胞毒性,IC 50 <10 μM。使用 goniodiol-7-monoacetate ( 3 )对 MDA-MB-231 细胞进行细胞周期分析和膜联蛋白-V 测定,显示细胞凋亡反应和坏死反应,并显示细胞增殖停滞在 G2/M 期。通过共价对接与 α-微管蛋白靶标进行了这些分子的计算机靶标鉴定。为了深入了解并确定这些蛋白质-配体复合物在热力学能级上的稳定性,基于通过 DFT 研究进行的反应能量分析,对与 α-微管蛋白的 Cys-316 结合的分离分子进行了进一步评估,这表明分离出的分子可能是类似于 Pironetin 的 α-微管蛋白抑制剂。分子动力学重申了观察结果。
更新日期:2023-07-21
中文翻译:

Goniothalamus wynaadensis Bedd 植物化学成分的分离、结构解析和体外抗癌活性。通过计算机模拟研究鉴定 α-微管蛋白作为假定的分子靶标
Goniothalamus wynaadensis Bedd的乙酸乙酯和甲醇提取物的植物化学分析。叶子导致分离出八 ( 1 – 8 ) 个已知分子,其中七 ( 2 – 8 ) 是首次从该物种中分离出来,其中包括 (+)-goniothalamin 氧化物 ( 2 )、goniodiol-7-monoacetate ( 3 )、gonidiol-8-monoacetate ( 4 )、goniodiol ( 5 )、(+)-8-epi-9-deoxygoniopypyrone ( 6 ) 等。通过乙酰化3和4进行植物化学修饰,得到 gonidiol diacetate ( 9 ),其绝对值通过单晶X射线衍射证实了构型(6R , 7R , 8R ) 。化合物3 – 9对乳腺癌、卵巢癌、前列腺癌和结肠癌细胞系具有细胞毒性,IC 50 <10 μM。使用 goniodiol-7-monoacetate ( 3 )对 MDA-MB-231 细胞进行细胞周期分析和膜联蛋白-V 测定,显示细胞凋亡反应和坏死反应,并显示细胞增殖停滞在 G2/M 期。通过共价对接与 α-微管蛋白靶标进行了这些分子的计算机靶标鉴定。为了深入了解并确定这些蛋白质-配体复合物在热力学能级上的稳定性,基于通过 DFT 研究进行的反应能量分析,对与 α-微管蛋白的 Cys-316 结合的分离分子进行了进一步评估,这表明分离出的分子可能是类似于 Pironetin 的 α-微管蛋白抑制剂。分子动力学重申了观察结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号