当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Scalable Processes for the Preparation of 4-(chloromethyl)-1-cyclohexyl-2-(trifluoromethyl)benzene: A Key Intermediate for Siponimod
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-07-21 , DOI: 10.1021/acs.oprd.3c00170 Biyue Lin 1, 2, 3 , Shuming Wu 2, 3 , Qingbo Xiao 2 , Jingping Kou 2 , Ji’an Hu 2 , Zhu Zhu 2 , Xinglin Zhou 2 , Jiang Weng 1 , Zhongqing Wang 2, 3, 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-07-21 , DOI: 10.1021/acs.oprd.3c00170 Biyue Lin 1, 2, 3 , Shuming Wu 2, 3 , Qingbo Xiao 2 , Jingping Kou 2 , Ji’an Hu 2 , Zhu Zhu 2 , Xinglin Zhou 2 , Jiang Weng 1 , Zhongqing Wang 2, 3, 4
Affiliation
|
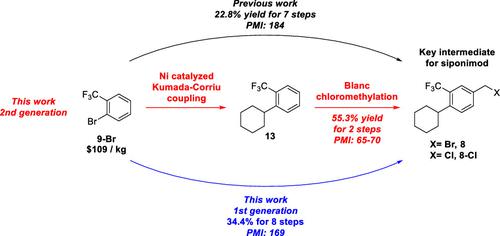
This paper presents the development of two generations of routes for the synthesis of the key intermediate 8-Cl of siponimod. The first generation focuses on a cyanation reaction followed by alkaline hydrolysis to introduce the benzoic acid group, replacing the hazardous nucleophilic carboxylation mediated by n-BuLi in the reported manufacturing route. Furthermore, the use of LiAlH4 for the carboxylic acid reduction is substituted with a milder acid anhydride reduction enabled by NaBH4. Overall, the first-generation route demonstrates an 11.6% increase in yield over 8 steps, effectively addressing concerns related to scale-up effects and safety-critical operations. In the second generation, a two-step synthesis involving nickel-catalyzed Kumada–Corriu coupling and Blanc chloromethylation is devised to produce benzyl chloride 8-Cl, starting from the readily available and cost-effective material 1-halo-2-(trifluoromethyl)benzene 9. The second-generation route is successfully demonstrated at large scales ranging from hundreds to kilo grams, resulting in a remarkable 32.5% yield increase and approximately 65% reduction in process mass intensity for the synthesis of intermediate 8-Cl.
中文翻译:

开发用于制备 4-(氯甲基)-1-环己基-2-(三氟甲基)苯的可扩展工艺:西波尼莫德的关键中间体
本文介绍了辛波尼莫德关键中间体8-Cl的两代合成路线的开发。第一代侧重于氰化反应,然后进行碱性水解以引入苯甲酸基团,取代了报道的制造路线中由正丁基锂介导的危险的亲核羧化反应。此外,使用 LiAlH 4进行羧酸还原被 NaBH 4实现的更温和的酸酐还原取代。总体而言,第一代路线在 8 个步骤中产量提高了 11.6%,有效解决了与放大效应和安全关键操作相关的问题。在第二代中,设计了一种两步合成法,涉及镍催化的 Kumada-Corriu 偶联和 Blanc 氯甲基化,以易于获得且具有成本效益的材料 1-halo-2-(三氟甲基)苯9为原料,生产氯化苄8-Cl。第二代路线在从数百克到公斤级的规模上成功进行了验证,使中间体 8-Cl 的合成收率显着提高了 32.5%,工艺质量强度降低了约 65 %。
更新日期:2023-07-22
中文翻译:

开发用于制备 4-(氯甲基)-1-环己基-2-(三氟甲基)苯的可扩展工艺:西波尼莫德的关键中间体
本文介绍了辛波尼莫德关键中间体8-Cl的两代合成路线的开发。第一代侧重于氰化反应,然后进行碱性水解以引入苯甲酸基团,取代了报道的制造路线中由正丁基锂介导的危险的亲核羧化反应。此外,使用 LiAlH 4进行羧酸还原被 NaBH 4实现的更温和的酸酐还原取代。总体而言,第一代路线在 8 个步骤中产量提高了 11.6%,有效解决了与放大效应和安全关键操作相关的问题。在第二代中,设计了一种两步合成法,涉及镍催化的 Kumada-Corriu 偶联和 Blanc 氯甲基化,以易于获得且具有成本效益的材料 1-halo-2-(三氟甲基)苯9为原料,生产氯化苄8-Cl。第二代路线在从数百克到公斤级的规模上成功进行了验证,使中间体 8-Cl 的合成收率显着提高了 32.5%,工艺质量强度降低了约 65 %。











































 京公网安备 11010802027423号
京公网安备 11010802027423号