Cell Chemical Biology ( IF 6.6 ) Pub Date : 2023-07-20 , DOI: 10.1016/j.chembiol.2023.06.018 Paschalis-Thomas Doulias 1 , Hongmei Yang 2 , Alexander Y Andreyev 3 , Nima Dolatabadi 3 , Henry Scott 3 , Charlene K Raspur 3 , Parth R Patel 3 , Tomohiro Nakamura 3 , Steven R Tannenbaum 4 , Harry Ischiropoulos 5 , Stuart A Lipton 6
|
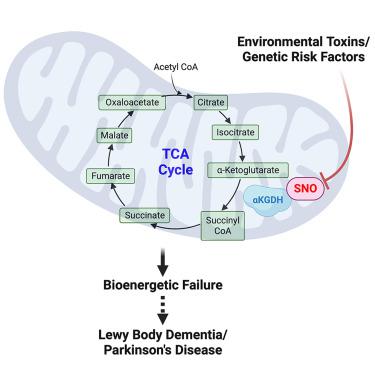
A causal relationship between mitochondrial metabolic dysfunction and neurodegeneration has been implicated in synucleinopathies, including Parkinson disease (PD) and Lewy body dementia (LBD), but underlying mechanisms are not fully understood. Here, using human induced pluripotent stem cell (hiPSC)-derived neurons with mutation in the gene encoding α-synuclein (αSyn), we report the presence of aberrantly S-nitrosylated proteins, including tricarboxylic acid (TCA) cycle enzymes, resulting in activity inhibition assessed by carbon-labeled metabolic flux experiments. This inhibition principally affects α-ketoglutarate dehydrogenase/succinyl coenzyme-A synthetase, metabolizing α-ketoglutarate to succinate. Notably, human LBD brain manifests a similar pattern of aberrantly S-nitrosylated TCA enzymes, indicating the pathophysiological relevance of these results. Inhibition of mitochondrial energy metabolism in neurons is known to compromise dendritic length and synaptic integrity, eventually leading to neuronal cell death. Our evidence indicates that aberrant S-nitrosylation of TCA cycle enzymes contributes to this bioenergetic failure.
中文翻译:

在死后人脑和 hiPSC 衍生神经元中研究的突触核蛋白病中 S-亚硝基化介导的 TCA 循环酶功能障碍
线粒体代谢功能障碍和神经退行性变之间的因果关系与突触核蛋白病有关,包括帕金森病(PD)和路易体痴呆(LBD),但其潜在机制尚不完全清楚。在这里,使用编码 α-突触核蛋白 (αSyn) 的基因发生突变的人诱导多能干细胞 (hiPSC) 衍生的神经元,我们报告了异常 S-亚硝基化蛋白的存在,包括三羧酸 (TCA) 循环酶,从而导致活性通过碳标记代谢流实验评估抑制作用。这种抑制主要影响 α-酮戊二酸脱氢酶/琥珀酰辅酶-A 合成酶,将 α-酮戊二酸代谢为琥珀酸。值得注意的是,人类 LBD 大脑表现出类似的异常 S-亚硝基化 TCA 酶模式,表明这些结果的病理生理学相关性。已知抑制神经元线粒体能量代谢会损害树突长度和突触完整性,最终导致神经元细胞死亡。我们的证据表明 TCA 循环酶的异常 S-亚硝基化导致了这种生物能失败。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号