Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-07-19 , DOI: 10.1016/j.actbio.2023.07.016
Mengxin Wang 1 , Xue Zhang 1 , Qian Chang 1 , Haifeng Zhang 1 , Zhenbo Zhang 2 , Kailin Li 1 , Hui Liu 1 , Donglin Liu 1 , Lu An 1 , Qiwei Tian 3
|
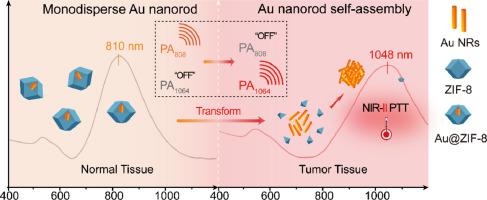
The misdiagnosis of tumors due to insufficient penetration depth or signal interference and damage to normal tissues due to indiscriminate treatment are the biggest challenges in using photothermal agents for clinical translation. To overcome these limitations, a strategy of switching from the near-infrared (NIR)-I region to the NIR-II region was developed based on tumor microenvironment (TME)-mediated gold (Au) self-assembly. Using zeolitic imidazolate framework-8 (ZIF-8) metal–organic framework-coated gold nanorods (AuNRs@ZIF-8) as a model photothermal agent, we demonstrated that only a NIR-I photoacoustic imaging signal was observed in normal tissue because ZIF-8 could prevent the aggregation of AuNRs. However, when ZIF-8 dissociated in the TME, the AuNRs aggregated to activate NIR-II photoacoustic imaging and attenuate the NIR-I signal, thereby allowing an accurate diagnosis of tumors based on signal transformation. Notably, TME-activated NIR-II photothermal therapy could also inhibit tumor growth. Therefore, this TME-activated NIR-I-to-NIR-II switching strategy could improve the accuracy of deep-tumor diagnoses and avoid the injury caused by undifferentiated treatment.
Statement of significance
Photothermal agents used for photoacoustic imaging and photothermal therapy have garnered great attention for tumor theranostics. However, always “turned on” near-infrared (NIR)-I laser (700–1000 nm)-responsive photothermal agents face issues of penetration depth and damage to normal tissues. In contrast, tumor microenvironment-activated NIR-II “smart” photothermal agents exhibit deeper penetration depth and tumor selectivity. Therefore, a NIR-I-to-NIR-II switching strategy was developed based on tumor microenvironment-mediated Au self-assembly. This work provides a new strategy for developing tumor microenvironment-activated NIR-II smart photothermal agents.
中文翻译:

肿瘤微环境介导的金自组装 NIR-I 到 NIR-II 转化用于治疗诊断
由于穿透深度不足或信号干扰而导致的肿瘤误诊以及由于不加区别的治疗而对正常组织造成的损害是使用光热制剂进行临床转化的最大挑战。为了克服这些限制,基于肿瘤微环境(TME)介导的金(Au)自组装开发了从近红外(NIR)-I区域切换到NIR-II区域的策略。使用沸石咪唑酯骨架-8(ZIF-8)金属有机骨架涂覆的金纳米棒(AuNRs@ZIF-8)作为模型光热剂,我们证明在正常组织中仅观察到 NIR-I 光声成像信号,因为 ZIF -8可以防止AuNRs的聚集。然而,当 ZIF-8 在 TME 中解离时,AuNR 聚集以激活 NIR-II 光声成像并减弱 NIR-I 信号,从而可以根据信号转换准确诊断肿瘤。值得注意的是,TME 激活的 NIR-II 光热疗法也可以抑制肿瘤生长。因此,这种TME激活的NIR-I到NIR-II转换策略可以提高深部肿瘤诊断的准确性,并避免未分化治疗造成的损伤。
重要性声明
用于光声成像和光热治疗的光热剂在肿瘤治疗诊断中引起了极大的关注。然而,始终“打开”的近红外 (NIR)-I 激光(700–1000 nm)响应光热剂面临着穿透深度和对正常组织损伤的问题。相比之下,肿瘤微环境激活的 NIR-II“智能”光热剂表现出更深的渗透深度和肿瘤选择性。因此,基于肿瘤微环境介导的Au自组装开发了NIR-I到NIR-II的转换策略。这项工作为开发肿瘤微环境激活的NIR-II智能光热剂提供了新策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号