当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacological and Physicochemical Properties Optimization for Dual-Target Dopamine D3 (D3R) and μ-Opioid (MOR) Receptor Ligands as Potentially Safer Analgesics
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-07-19 , DOI: 10.1021/acs.jmedchem.3c00417 Alessandro Bonifazi 1 , Elizabeth Saab 1 , Julie Sanchez 2, 3 , Antonina L Nazarova 4 , Saheem A Zaidi 4 , Khorshada Jahan 1 , Vsevolod Katritch 4 , Meritxell Canals 2, 3 , J Robert Lane 2, 3 , Amy Hauck Newman 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-07-19 , DOI: 10.1021/acs.jmedchem.3c00417 Alessandro Bonifazi 1 , Elizabeth Saab 1 , Julie Sanchez 2, 3 , Antonina L Nazarova 4 , Saheem A Zaidi 4 , Khorshada Jahan 1 , Vsevolod Katritch 4 , Meritxell Canals 2, 3 , J Robert Lane 2, 3 , Amy Hauck Newman 1
Affiliation
|
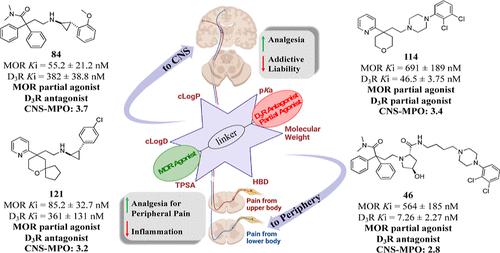
A new generation of dual-target μ opioid receptor (MOR) agonist/dopamine D3 receptor (D3R) antagonist/partial agonists with optimized physicochemical properties was designed and synthesized. Combining in vitro cell-based on-target/off-target affinity screening, in silico computer-aided drug design, and BRET functional assays, we identified new structural scaffolds that achieved high affinity and agonist/antagonist potencies for MOR and D3R, respectively, improving the dopamine receptor subtype selectivity (e.g., D3R over D2R) and significantly enhancing central nervous system multiparameter optimization scores for predicted blood–brain barrier permeability. We identified the substituted trans-(2S,4R)-pyrrolidine and trans-phenylcyclopropyl amine as key dopaminergic moieties and tethered these to different opioid scaffolds, derived from the MOR agonists TRV130 (3) or loperamide (6). The lead compounds 46, 84, 114, and 121 have the potential of producing analgesic effects through MOR partial agonism with reduced opioid-misuse liability via D3R antagonism. Moreover, the peripherally limited derivatives could have therapeutic indications for inflammation and neuropathic pain.
中文翻译:

双靶点多巴胺 D3 (D3R) 和 μ-阿片 (MOR) 受体配体作为潜在更安全镇痛药的药理和理化性质优化
设计合成了理化性质优化的新一代双靶点μ阿片受体(MOR)激动剂/多巴胺D 3受体(D 3 R)拮抗剂/部分激动剂。结合体外基于细胞的在靶/脱靶亲和力筛选、计算机辅助药物设计和 BRET 功能测定,我们确定了新的结构支架,可实现 MOR 和 D 3 R 的高亲和力和激动剂/拮抗剂效力,分别提高多巴胺受体亚型选择性(例如,D 3 R 优于 D 2 R)并显着提高预测血脑屏障渗透性的中枢神经系统多参数优化得分。我们确定了取代的反式-( 2S , 4R )-吡咯烷和反式-苯基环丙胺作为关键的多巴胺能部分,并将它们连接到源自 MOR 激动剂TRV130 ( 3 ) 或洛哌丁胺 ( 6 ) 的不同阿片类支架。先导化合物46、84、114和121具有通过MOR部分激动作用产生镇痛作用的潜力,并通过D 3 R拮抗作用减少阿片类药物滥用的可能性。此外,外周受限衍生物可能具有治疗炎症和神经性疼痛的适应症。
更新日期:2023-07-19
中文翻译:

双靶点多巴胺 D3 (D3R) 和 μ-阿片 (MOR) 受体配体作为潜在更安全镇痛药的药理和理化性质优化
设计合成了理化性质优化的新一代双靶点μ阿片受体(MOR)激动剂/多巴胺D 3受体(D 3 R)拮抗剂/部分激动剂。结合体外基于细胞的在靶/脱靶亲和力筛选、计算机辅助药物设计和 BRET 功能测定,我们确定了新的结构支架,可实现 MOR 和 D 3 R 的高亲和力和激动剂/拮抗剂效力,分别提高多巴胺受体亚型选择性(例如,D 3 R 优于 D 2 R)并显着提高预测血脑屏障渗透性的中枢神经系统多参数优化得分。我们确定了取代的反式-( 2S , 4R )-吡咯烷和反式-苯基环丙胺作为关键的多巴胺能部分,并将它们连接到源自 MOR 激动剂TRV130 ( 3 ) 或洛哌丁胺 ( 6 ) 的不同阿片类支架。先导化合物46、84、114和121具有通过MOR部分激动作用产生镇痛作用的潜力,并通过D 3 R拮抗作用减少阿片类药物滥用的可能性。此外,外周受限衍生物可能具有治疗炎症和神经性疼痛的适应症。






























 京公网安备 11010802027423号
京公网安备 11010802027423号