当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Annulated Spiro[4.4]-nonane-diones via the Tandem [4 + 2]-Cycloaddition/Aromatization Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-07-18 , DOI: 10.1021/acs.joc.3c00981 Konstantin S Ivanov 1 , Denis E Samburskiy 1 , Leila V Zargarova 1, 2 , Vladislav Yu Komarov 2 , Evgeny A Mostovich 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-07-18 , DOI: 10.1021/acs.joc.3c00981 Konstantin S Ivanov 1 , Denis E Samburskiy 1 , Leila V Zargarova 1, 2 , Vladislav Yu Komarov 2 , Evgeny A Mostovich 1
Affiliation
|
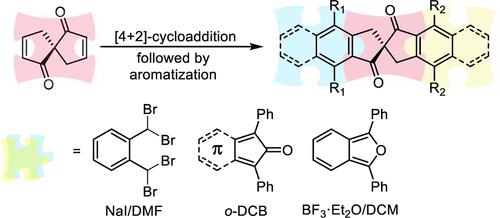
A method for the synthesis of both symmetric and asymmetric fused spiro[4.4]-nonane-dione derivatives has been developed. It is based on a Diels–Alder reaction of spiro[4.4]nona-2,7-diene-1,6-dione as a dienophile component followed by immediate aromatization of the adduct. An active diene component can be generated using the tetrabromoxylene/NaI system, the 1,3-diphenylisobenzofuran/BF3 system, or substituted cyclones.
中文翻译:

通过串联 [4 + 2]-环加成/芳构化反应构建环状螺[4.4]-壬烷二酮
开发了一种合成对称和不对称稠合螺[4.4]-壬烷二酮衍生物的方法。它基于螺[4.4]nona-2,7-二烯-1,6-二酮作为亲二烯体组分的狄尔斯-阿尔德反应,然后立即对加合物进行芳构化。活性二烯组分可以使用四溴二甲苯/NaI体系、1,3-二苯基异苯并呋喃/BF 3体系或取代旋风分离器来产生。
更新日期:2023-07-18
中文翻译:

通过串联 [4 + 2]-环加成/芳构化反应构建环状螺[4.4]-壬烷二酮
开发了一种合成对称和不对称稠合螺[4.4]-壬烷二酮衍生物的方法。它基于螺[4.4]nona-2,7-二烯-1,6-二酮作为亲二烯体组分的狄尔斯-阿尔德反应,然后立即对加合物进行芳构化。活性二烯组分可以使用四溴二甲苯/NaI体系、1,3-二苯基异苯并呋喃/BF 3体系或取代旋风分离器来产生。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号