当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogen Trifluoride Complexes of Group 10 Transition Metals M(NF3) (M = Pd, Pt)
Chemical Science ( IF 7.6 ) Pub Date : 2023-07-18 , DOI: 10.1039/d3sc02313c Guohai Deng 1 , Yan Lu 1 , Tony Stüker 1 , Sebastian Riedel 1
Chemical Science ( IF 7.6 ) Pub Date : 2023-07-18 , DOI: 10.1039/d3sc02313c Guohai Deng 1 , Yan Lu 1 , Tony Stüker 1 , Sebastian Riedel 1
Affiliation
|
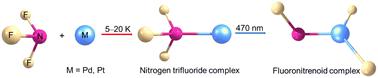
The group 10 transition metal atoms Pd and Pt react with nitrogen trifluoride (NF3) forming N-coordination M(NF3) complexes in solid neon and argon matrices. The M(NF3) complexes isomerize to more stable fluoronitrenoid FNMF2 isomers via fluorine migration upon blue LED (λ = 470 nm) light irradiation. These products are characterized on the basis of infrared absorption spectroscopy with isotopic substitutions and theoretical frequency calculations. The analysis of the electronic structure of nitrogen trifluoride complexes indicates that the bonding between metal and nitrogen trifluoride can be described as σ donation from the HOMO of nitrogen trifluoride to the empty metal dz2 orbital and π back-donation from the metal dxz/yz orbitals to the LUMO of nitrogen trifluoride, the latter of which stabilized the metal ligand bond and destabilized the ligand N–F bond. In FNMF2, the FN ligand doubly bonded to the metal and bear imido character.
中文翻译:

第 10 族过渡金属三氟化氮配合物 M(NF3) (M = Pd, Pt)
第 10 族过渡金属原子 Pd 和 Pt 与三氟化氮 (NF 3 ) 反应,在固体氖和氩基质中形成 N 配位 M(NF 3 ) 配合物。M(NF 3 ) 配合物在蓝色 LED ( λ = 470 nm) 光照射下通过氟迁移异构化为更稳定的氟氮化合物 FNMF 2异构体。这些产品基于具有同位素取代的红外吸收光谱和理论频率计算进行表征。对三氟化氮配合物电子结构的分析表明,金属与三氟化氮之间的键合可以描述为三氟化氮HOMO向空金属d z 2 轨道的σ供体和金属d xz / 的π反供体。yz轨道连接到三氟化氮的 LUMO,后者稳定了金属配体键,并使配体 N-F 键不稳定。在FNMF 2中,FN配体与金属双键结合并具有亚氨基特征。
更新日期:2023-07-18
中文翻译:

第 10 族过渡金属三氟化氮配合物 M(NF3) (M = Pd, Pt)
第 10 族过渡金属原子 Pd 和 Pt 与三氟化氮 (NF 3 ) 反应,在固体氖和氩基质中形成 N 配位 M(NF 3 ) 配合物。M(NF 3 ) 配合物在蓝色 LED ( λ = 470 nm) 光照射下通过氟迁移异构化为更稳定的氟氮化合物 FNMF 2异构体。这些产品基于具有同位素取代的红外吸收光谱和理论频率计算进行表征。对三氟化氮配合物电子结构的分析表明,金属与三氟化氮之间的键合可以描述为三氟化氮HOMO向空金属d z 2 轨道的σ供体和金属d xz / 的π反供体。yz轨道连接到三氟化氮的 LUMO,后者稳定了金属配体键,并使配体 N-F 键不稳定。在FNMF 2中,FN配体与金属双键结合并具有亚氨基特征。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号