Applied Surface Science ( IF 6.3 ) Pub Date : 2023-07-17 , DOI: 10.1016/j.apsusc.2023.158056 Yujing Han , Huixiang Wang , Dongmei Huang , Pengfei Wang , Jianli Zhang , Xiaobo Ren , Yu Meng , Baoliang Lv
|
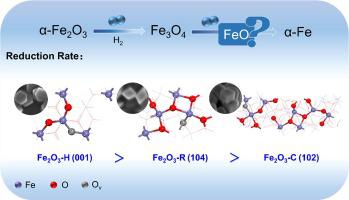
The reduction of α-Fe2O3 is significant in heterogeneous catalysis. However, due to the changeable crystal phase and the transformation of crystal facet, its reduction mechanism still needs further clarification. In this work, α-Fe2O3 rhombohedra, hexagonal-plate and pseudo-cubic with the facets of (1 0 4), (0 0 1) and (1 0 2) respectively were synthesized, and the evolutions of α-Fe2O3 structures during H2 reduction were investigated by multiple means including in-situ XRD. It found that the reduction of α-Fe2O3 showed a facet-dependent behavior in H2 atmosphere, that is different facets exhibited completely different rates and phase transition pathways. Specifically, the Fe2O3 (1 0 4) and Fe3O4 (2 2 0) had the fastest reduction rate in α-Fe2O3 → Fe3O4 and Fe3O4 → α-Fe, respectively, which was attributed to the higher concentration of oxygen vacancies on the corresponding crystal facets. The phase transition paths for Fe2O3 (1 0 2) and Fe2O3 (1 0 4) were α-Fe2O3 → Fe3O4 → α-Fe, while Fe2O3 (0 0 1) was α-Fe2O3 → Fe3O4 → FeO → α-Fe. Additionally, DFT calculations further revealed that Fe2O3 (1 0 4) and Fe3O4 (2 2 0) had lower interaction energy between O and Fe atoms and easily formed oxygen vacancy. Consequently, these facets presented different formation rates of H2O, leading to significant differences in the phase transition process.
中文翻译:

氢气氛中 α-Fe2O3 晶面依赖性还原行为
α-Fe 2 O 3的还原在多相催化中具有重要意义。但由于晶相的变化和晶面的转变,其还原机理仍需进一步阐明。本工作合成了面分别为(1 0 4)、(0 0 1)和(1 0 2)的α-Fe 2 O 3菱面体、六方板和赝立方体,并研究了α-Fe 2 O 3 的演化过程。通过包括原位XRD在内的多种手段研究了H 2还原过程中Fe 2 O 3 的结构。结果发现,α-Fe 2 O 3的还原 在H 2气氛中表现出依赖于面的行为,即不同面表现出完全不同的速率和相变路径。具体而言,Fe 2 O 3 (1 0 4)和Fe 3 O 4 (2 2 0)分别在α-Fe 2 O 3 → Fe 3 O 4和Fe 3 O 4 → α-Fe 中具有最快的还原率。 ,这归因于相应晶面上氧空位的浓度较高。Fe 2 O 3 (1 0 2) 和 Fe的相变路径 2 O 3 (1 0 4) 为 α-Fe 2 O 3 → Fe 3 O 4 → α-Fe,而 Fe 2 O 3 (0 0 1) 为 α-Fe 2 O 3 → Fe 3 O 4 → FeO → α-Fe。此外,DFT计算进一步表明,Fe 2 O 3 (1 0 4)和Fe 3 O 4 (2 2 0)的O和Fe原子之间的相互作用能较低,容易形成氧空位。因此,这些方面呈现出不同的 H 2形成速率O,导致相变过程显着差异。































 京公网安备 11010802027423号
京公网安备 11010802027423号