Chem Catalysis ( IF 11.5 ) Pub Date : 2023-07-14 , DOI: 10.1016/j.checat.2023.100697 Bing-ru Shao , Wen-feng Jiang , Chao Zheng , Lei Shi
|
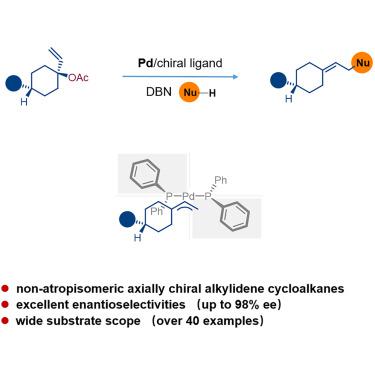
Precise synthesis of axially chiral molecules is an important goal that organic chemists are actively pursuing because of their high value in pharmaceutics, fine chemicals, and materials science. The enantioselective synthesis of non-atropisomeric axially chiral alkylidene cycloalkanes, despite their utility, has been considered a great challenge and remains underexplored due to the remote distance between the chirality-related groups or atoms. We established a general efficient methodology to synthesize axially chiral alkylidene cycloalkanes via palladium-catalyzed asymmetric allylic alkylation, showing excellent enantioselectivities, high yields, and a wide substrate scope. The additive effect plays a pivotal role to improve the chem- and enantioselectivity in this reaction. Due to the value and structural diversity of the products, this method provides the opportunity to further tap into the applied potential of axially chiral olefins in related research fields.
中文翻译:

Pd催化对映选择性合成轴向手性亚烷基环烷烃
轴向手性分子的精确合成是有机化学家积极追求的一个重要目标,因为它们在制药、精细化学品和材料科学中具有很高的价值。非阻转异构轴向手性亚烷基环烷烃的对映选择性合成尽管具有实用性,但仍被认为是一个巨大的挑战,并且由于手性相关基团或原子之间的距离较远而仍未得到充分探索。我们建立了一种通过钯催化的不对称烯丙基烷基化合成轴向手性亚烷基环烷烃的通用有效方法,表现出优异的性能对映选择性、高产率和广泛的底物范围。加成效应对于提高该反应的化学选择性和对映选择性起着关键作用。由于产物的价值和结构多样性,该方法为进一步挖掘轴向手性烯烃在相关研究领域的应用潜力提供了机会。






























 京公网安备 11010802027423号
京公网安备 11010802027423号