当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced removal of Ni-EDTA by three-dimensional electro-Fenton process using Fe-N-doped biochar as catalytic particle electrodes
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-07-14 , DOI: 10.1016/j.cej.2023.144717
Zilong Zhao , Yatao Ren , Shuyu Qi , Zigong Ning , Xing Wang , Wenyi Dong , Hongjie Wang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-07-14 , DOI: 10.1016/j.cej.2023.144717
Zilong Zhao , Yatao Ren , Shuyu Qi , Zigong Ning , Xing Wang , Wenyi Dong , Hongjie Wang
|
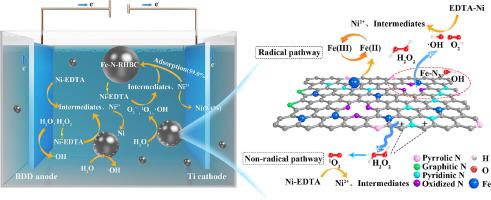
Heavy metal complexes pose a challenge in terms of their resistance to removal due to their stable and intricate structure. In this study, we explored the utilization of Fe and N co-doped biochar derived from rice husk (Fe-N-RHBC) as catalytic particle electrodes in a three-dimensional electro-Fenton (3D-EF) system for the decomplexation and removal of a model pollutant, Ni-EDTA. The characterization analysis and comparison of catalytic activities confirmed the beneficial effects of Fe and N co-doping on various aspects, such as the types of active sites, interfacial electron transfer properties, and H2 O2 decomposition capacity. Compared to other investigated oxidation systems, the 3D-EF process demonstrated rapid removal of Ni-EDTA within the initial minute, with a maximum H2 O2 utilization of 99.1% after 30 min. The prepared catalytic particle electrodes exhibited high stability and catalytic capacity even after three consecutive cycles. Throughout the reaction, several processes, including anode oxidation, particle electrode polarization, catalysis, and adsorption of Fe-N-RHBC, as well as cathodic electro-deposition, synergistically contributed to the treatment of Ni-EDTA. The surface-bound O2 1 O2 generated by the Fe-N-RHBC catalytic particle electrode played a crucial role in the decomplexation and removal of Ni-EDTA. Building upon these findings, we further proposed potential mechanisms for the removal of Ni-EDTA in the 3D-EF system using the Fe-N-RHBC catalytic particle electrode.
中文翻译:

使用 Fe-N 掺杂生物炭作为催化颗粒电极,通过三维电 Fenton 工艺增强对 Ni-EDTA 的去除
由于其稳定而复杂的结构,重金属络合物在抗氧化性方面提出了挑战。在这项研究中,我们探索了利用稻壳衍生的 Fe 和 N 共掺杂生物炭 (Fe-N-RHBC) 作为三维电芬顿 (3D-EF) 系统中的催化粒子电极,用于解络和去除模型污染物 Ni-EDTA。催化活性的表征分析和比较证实了 Fe 和 N 共掺杂对活性位点类型、界面电子转移性质和 H2O2 分解能力等各个方面的有益影响。与其他研究的氧化系统相比,3D-EF 工艺在初始分钟内快速去除了 Ni-EDTA,30 分钟后 H2O2 的最大利用率为 99.1%。所制备的催化颗粒电极即使在连续三次循环后也表现出高稳定性和催化能力。在整个反应过程中,包括阳极氧化、颗粒电极极化、催化和 Fe-N-RHBC 吸附以及阴极电沉积在内的几个过程协同促进了 Ni-EDTA 的处理。Fe-N-RHBC 催化颗粒电极产生的表面结合 O2− 自由基和 1O2 在 Ni-EDTA 的解络和去除中起着至关重要的作用。基于这些发现,我们进一步提出了使用 Fe-N-RHBC 催化粒子电极在 3D-EF 系统中去除 Ni-EDTA 的潜在机制。
更新日期:2023-07-14
中文翻译:

使用 Fe-N 掺杂生物炭作为催化颗粒电极,通过三维电 Fenton 工艺增强对 Ni-EDTA 的去除
由于其稳定而复杂的结构,重金属络合物在抗氧化性方面提出了挑战。在这项研究中,我们探索了利用稻壳衍生的 Fe 和 N 共掺杂生物炭 (Fe-N-RHBC) 作为三维电芬顿 (3D-EF) 系统中的催化粒子电极,用于解络和去除模型污染物 Ni-EDTA。催化活性的表征分析和比较证实了 Fe 和 N 共掺杂对活性位点类型、界面电子转移性质和 H2O2 分解能力等各个方面的有益影响。与其他研究的氧化系统相比,3D-EF 工艺在初始分钟内快速去除了 Ni-EDTA,30 分钟后 H2O2 的最大利用率为 99.1%。所制备的催化颗粒电极即使在连续三次循环后也表现出高稳定性和催化能力。在整个反应过程中,包括阳极氧化、颗粒电极极化、催化和 Fe-N-RHBC 吸附以及阴极电沉积在内的几个过程协同促进了 Ni-EDTA 的处理。Fe-N-RHBC 催化颗粒电极产生的表面结合 O2− 自由基和 1O2 在 Ni-EDTA 的解络和去除中起着至关重要的作用。基于这些发现,我们进一步提出了使用 Fe-N-RHBC 催化粒子电极在 3D-EF 系统中去除 Ni-EDTA 的潜在机制。




































 京公网安备 11010802027423号
京公网安备 11010802027423号