当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific Glycation of Human Heat Shock Protein (Hsp27) Enhances Its Chaperone Activity
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2023-07-14 , DOI: 10.1021/acschembio.3c00214
Somnath Mukherjee 1 , Dominik P Vogl 1, 2 , Christian F W Becker 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2023-07-14 , DOI: 10.1021/acschembio.3c00214
Somnath Mukherjee 1 , Dominik P Vogl 1, 2 , Christian F W Becker 1
Affiliation
|
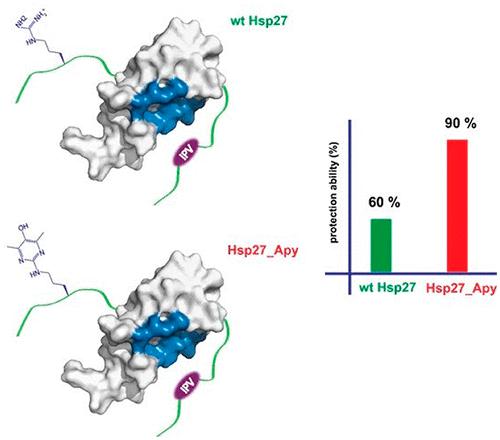
Non-enzymatic posttranslational modifications are believed to affect at least 30% of human proteins, commonly termed glycation. Many of these modifications are implicated in various pathological conditions, e.g., cataract, diabetes, neurodegenerative diseases, and cancer. Chemical protein synthesis enables access to full-length proteins carrying site-specific modifications. One such modification, argpyrimidine (Apy), has been detected in human small heat shock protein Hsp27 and closely related proteins in patient-derived tissues. Thus far, studies have looked into only artificial mixtures of Apy modifications, and only one has analyzed Apy188. We were interested in understanding the impact of such individual Apy modifications on five different arginine sites within the crucial N-terminal domain of Hsp27. By combining protein semisynthesis with biochemical assays on semisynthetic Hsp27 analogues with single-point Apy modification at those sites, we have shown how a seemingly minimal modification within this region results in dramatically altered functional attributes.
中文翻译:

人热休克蛋白 (Hsp27) 的位点特异性糖化增强其伴侣活性
据信非酶翻译后修饰会影响至少 30% 的人类蛋白质,通常称为糖化。许多这些修饰与各种病理状况有关,例如白内障、糖尿病、神经退行性疾病和癌症。化学蛋白质合成能够获得携带位点特异性修饰的全长蛋白质。一种这样的修饰,argpyrimidine (Apy),已在人类小热休克蛋白 Hsp27 和患者来源组织中密切相关的蛋白中检测到。迄今为止,研究仅研究了 Apy 修饰的人工混合物,并且只有一项研究分析了 Apy188。我们有兴趣了解此类单独的 Apy 修饰对 Hsp27 关键 N 末端结构域内五个不同精氨酸位点的影响。通过将蛋白质半合成与半合成 Hsp27 类似物的生化测定相结合,并在这些位点进行单点 Apy 修饰,我们已经展示了该区域内看似最小的修饰如何导致功能属性的显着改变。
更新日期:2023-07-14
中文翻译:

人热休克蛋白 (Hsp27) 的位点特异性糖化增强其伴侣活性
据信非酶翻译后修饰会影响至少 30% 的人类蛋白质,通常称为糖化。许多这些修饰与各种病理状况有关,例如白内障、糖尿病、神经退行性疾病和癌症。化学蛋白质合成能够获得携带位点特异性修饰的全长蛋白质。一种这样的修饰,argpyrimidine (Apy),已在人类小热休克蛋白 Hsp27 和患者来源组织中密切相关的蛋白中检测到。迄今为止,研究仅研究了 Apy 修饰的人工混合物,并且只有一项研究分析了 Apy188。我们有兴趣了解此类单独的 Apy 修饰对 Hsp27 关键 N 末端结构域内五个不同精氨酸位点的影响。通过将蛋白质半合成与半合成 Hsp27 类似物的生化测定相结合,并在这些位点进行单点 Apy 修饰,我们已经展示了该区域内看似最小的修饰如何导致功能属性的显着改变。

































 京公网安备 11010802027423号
京公网安备 11010802027423号