当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiourea-Based Bifunctional Catalysts for Epoxide-Involved Ring-Opening Polymerization of α-Amino Acid N-Carboxyanhydrides
Macromolecules ( IF 5.1 ) Pub Date : 2023-07-14 , DOI: 10.1021/acs.macromol.3c00830
Kai-Yue Wang 1 , Zhuo-Qun Li 1 , Ming-Nuo Xu 1 , Bo Li 1
Macromolecules ( IF 5.1 ) Pub Date : 2023-07-14 , DOI: 10.1021/acs.macromol.3c00830
Kai-Yue Wang 1 , Zhuo-Qun Li 1 , Ming-Nuo Xu 1 , Bo Li 1
Affiliation
|
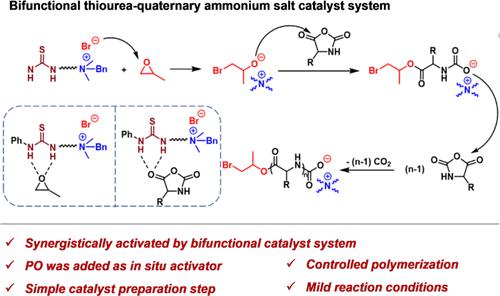
Thiourea-quaternary ammonium salt bifunctional catalysts with both electrophilic and nucleophilic centers were designed in this paper to initiate the ring-opening polymerization (ROP) of α-amino acid N-carboxyanhydrides (NCAs) for the efficient synthesis of polypeptides. We disclosed this kind of bifunctional catalyst for the efficient synthesis of polypeptides in the presence of propylene oxide (PO), which was judiciously added as an in situ activator for the efficient ROP of NCA. By MALDI-TOF MS and 1H NMR analysis, we identified the end groups of the polymer and clarified the ROP process in the presence of the bifunctional catalyst. We showed that the PO was initially ring-opened by the nucleophilic attack of a halogen ion; then, the newly formed alkoxide ions attacked the electrophilic carbonyl of NCA, providing the carbamate anion. After undergoing a decarboxylation of the carbamate anion, the nucleophilic attack of N to the carbonyl on an NCA ring occurred synergistically to complete the chain growth. The involved three steps formed the whole catalytic cycle. We also disclosed that the structural factors, including the electronic effect on electrophilic/nucleophilic centers, steric factors, and the coupling thermodynamics, have a strong relationship with catalyst performance. We believe the insightful structure–performance relationship will shed light on further metal-free catalyst design for artificial polypeptide synthesis.
中文翻译:

用于环氧化物参与的 α-氨基酸 N-羧酸酐开环聚合的硫脲基双功能催化剂
本文设计了具有亲电和亲核中心的硫脲-季铵盐双功能催化剂,用于引发α-氨基酸N-羧酸酐(NCAs)的开环聚合(ROP),从而实现多肽的高效合成。我们公开了这种在环氧丙烷(PO)存在下有效合成多肽的双功能催化剂,它被明智地添加作为NCA有效ROP的原位活化剂。通过 MALDI-TOF MS 和1通过 H NMR 分析,我们鉴定了聚合物的端基并阐明了双功能催化剂存在下的 ROP 过程。我们发现 PO 最初是通过卤素离子的亲核攻击而开环的;然后,新形成的醇盐离子攻击NCA的亲电羰基,提供氨基甲酸根阴离子。氨基甲酸酯阴离子脱羧后,N对NCA环上的羰基的亲核攻击协同发生,以完成链增长。这三个步骤构成了整个催化循环。我们还揭示了结构因素,包括电子对亲电/亲核中心的影响、空间因素和耦合热力学,与催化剂性能有很强的关系。
更新日期:2023-07-14
中文翻译:

用于环氧化物参与的 α-氨基酸 N-羧酸酐开环聚合的硫脲基双功能催化剂
本文设计了具有亲电和亲核中心的硫脲-季铵盐双功能催化剂,用于引发α-氨基酸N-羧酸酐(NCAs)的开环聚合(ROP),从而实现多肽的高效合成。我们公开了这种在环氧丙烷(PO)存在下有效合成多肽的双功能催化剂,它被明智地添加作为NCA有效ROP的原位活化剂。通过 MALDI-TOF MS 和1通过 H NMR 分析,我们鉴定了聚合物的端基并阐明了双功能催化剂存在下的 ROP 过程。我们发现 PO 最初是通过卤素离子的亲核攻击而开环的;然后,新形成的醇盐离子攻击NCA的亲电羰基,提供氨基甲酸根阴离子。氨基甲酸酯阴离子脱羧后,N对NCA环上的羰基的亲核攻击协同发生,以完成链增长。这三个步骤构成了整个催化循环。我们还揭示了结构因素,包括电子对亲电/亲核中心的影响、空间因素和耦合热力学,与催化剂性能有很强的关系。

































 京公网安备 11010802027423号
京公网安备 11010802027423号