当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Conductivity in LISICON Analogues within the Li4GeO4–Li2MoO4 System
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-07-14 , DOI: 10.1021/acs.inorgchem.3c01222
Ludan Zhang 1, 2 , Marcin Malys 3 , Jan Jamroz 3 , Franciszek Krok 3 , Wojciech Wrobel 3 , Stephen Hull 4 , Haixue Yan 5 , Isaac Abrahams 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-07-14 , DOI: 10.1021/acs.inorgchem.3c01222
Ludan Zhang 1, 2 , Marcin Malys 3 , Jan Jamroz 3 , Franciszek Krok 3 , Wojciech Wrobel 3 , Stephen Hull 4 , Haixue Yan 5 , Isaac Abrahams 1
Affiliation
|
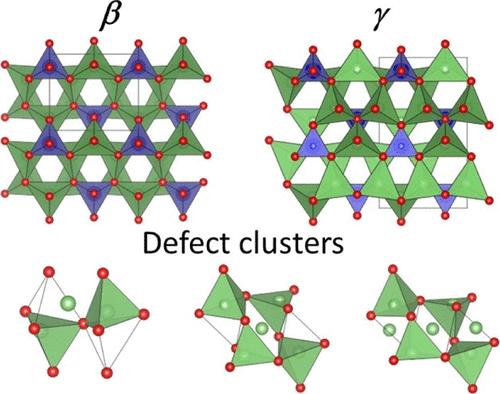
New solid electrolytes are crucial for the development of all-solid-state lithium batteries with advantages in safety and energy densities over current liquid electrolyte systems. While some of the best solid-state Li+-ion conductors are based on sulfides, their air sensitivity makes them less commercially attractive, and attention is refocusing on air-stable oxide-based systems. Among these, the LISICON-structured systems, such as Li2+2xZn1–xGeO4 and Li3+xV1–xGexO4, have been relatively well studied. However, other systems such as the Li4GeO4–Li2MoO4 system, which also show LISICON-type structures, have been relatively little explored. In this work, the Li4–2xGe1–xMoxO4 solid solution is investigated systematically, including the solid solution limit, structural stability, local structure, and the corresponding electrical behavior. It is found that a γ-LISICON structured solution is formed in the range of 0.1 ≤ x < 0.4, differing in structure from the two end members, Li4GeO4 and Li2MoO4. With increasing Mo content, the β-phase becomes increasingly more stable than the γ-phase, and at x = 0.5, a pure β-phase (β-Li3Ge0.5Mo0.5O4) is readily isolated. The structure of this previously unknown compound is presented, along with details of the defect structure of Li3.6Ge0.8Mo0.2O4 (x = 0.2) based on neutron diffraction data. Two basic types of defects are identified in Li3.6Ge0.8Mo0.2O4 involving interstitial Li+-ions in octahedral sites, with evidence for these coming together to form larger defect clusters. The x = 0.2 composition shows the highest conductivity of the series, with values of 1.11 × 10–7 S cm–1 at room temperature rising to 5.02 × 10–3 S cm–1 at 250 °C.
中文翻译:

Li4GeO4–Li2MoO4 系统中 LISICON 类似物的结构和电导率
新型固体电解质对于全固态锂电池的发展至关重要,其在安全性和能量密度方面比现有液体电解质系统具有优势。虽然一些最好的固态锂离子导体是基于硫化物的,但它们的空气敏感性使其商业吸引力较低,人们的注意力重新集中在空气稳定的基于氧化物的系统上。其中,LISICON结构的体系,如Li 2+2 x Zn 1– x GeO 4和Li 3+ x V 1– x Ge x O 4,已经得到了相对较好的研究。然而,其他体系,例如也显示LISICON型结构的Li 4 GeO 4 –Li 2 MoO 4体系,研究相对较少。在这项工作中,系统地研究了Li 4–2 x Ge 1– x Mo x O 4固溶体,包括固溶极限、结构稳定性、局部结构和相应的电学行为。发现在0.1≤x<0.4范围内形成γ-LISICON结构溶液,其结构与两个端元Li 4 GeO 4和Li 2 MoO 4不同。随着Mo含量的增加,β相变得比γ相更加稳定,并且在x =0.5时,很容易分离出纯β相(β-Li 3 Ge 0.5 Mo 0.5 O 4 )。介绍了这种以前未知的化合物的结构,以及基于中子衍射数据的 Li 3.6 Ge 0.8 Mo 0.2 O 4 ( x = 0.2) 缺陷结构的详细信息。在Li 3.6 Ge 0.8 Mo 0.2 O 4中发现了两种基本类型的缺陷,涉及八面体位点中的间隙Li + -离子,有证据表明这些缺陷聚集在一起形成更大的缺陷簇。x = 0.2 的组合物显示出该系列中最高的电导率,室温下的值为 1.11 × 10 –7 S cm –1,250 °C时为 5.02 × 10 –3 S cm –1 。
更新日期:2023-07-14
中文翻译:

Li4GeO4–Li2MoO4 系统中 LISICON 类似物的结构和电导率
新型固体电解质对于全固态锂电池的发展至关重要,其在安全性和能量密度方面比现有液体电解质系统具有优势。虽然一些最好的固态锂离子导体是基于硫化物的,但它们的空气敏感性使其商业吸引力较低,人们的注意力重新集中在空气稳定的基于氧化物的系统上。其中,LISICON结构的体系,如Li 2+2 x Zn 1– x GeO 4和Li 3+ x V 1– x Ge x O 4,已经得到了相对较好的研究。然而,其他体系,例如也显示LISICON型结构的Li 4 GeO 4 –Li 2 MoO 4体系,研究相对较少。在这项工作中,系统地研究了Li 4–2 x Ge 1– x Mo x O 4固溶体,包括固溶极限、结构稳定性、局部结构和相应的电学行为。发现在0.1≤x<0.4范围内形成γ-LISICON结构溶液,其结构与两个端元Li 4 GeO 4和Li 2 MoO 4不同。随着Mo含量的增加,β相变得比γ相更加稳定,并且在x =0.5时,很容易分离出纯β相(β-Li 3 Ge 0.5 Mo 0.5 O 4 )。介绍了这种以前未知的化合物的结构,以及基于中子衍射数据的 Li 3.6 Ge 0.8 Mo 0.2 O 4 ( x = 0.2) 缺陷结构的详细信息。在Li 3.6 Ge 0.8 Mo 0.2 O 4中发现了两种基本类型的缺陷,涉及八面体位点中的间隙Li + -离子,有证据表明这些缺陷聚集在一起形成更大的缺陷簇。x = 0.2 的组合物显示出该系列中最高的电导率,室温下的值为 1.11 × 10 –7 S cm –1,250 °C时为 5.02 × 10 –3 S cm –1 。

































 京公网安备 11010802027423号
京公网安备 11010802027423号