当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
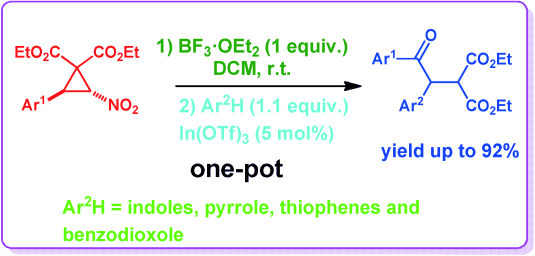
Boron Trifluoride‐Promoted Indium(III) Triflate‐Catalyzed Sequential One‐Pot Synthesis of (1,2‐Diaryl‐2‐oxoethyl)malonates from trans‐2‐Aryl‐3‐nitrocyclopropane‐1,1‐dicarboxylates and Activated Arenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-06-10 , DOI: 10.1002/adsc.201500143
Thangavel Selvi , Kannupal Srinivasan
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-06-10 , DOI: 10.1002/adsc.201500143
Thangavel Selvi , Kannupal Srinivasan
|
A sequential one‐pot synthesis of Michael adducts of aroylmethylidenemalonates with activated aromatics is described. The method involves treatment of trans‐2‐aryl‐3‐nitro‐cyclopropane‐1,1‐dicarboxylates with boron trifluoride etherate to form aroylmethylidenemalonates in situ and then addition of activated aromatics such as indoles, carbazole, pyrrole, thiophenes, methoxybenzenes and benzodioxole followed by a catalytic amount of indium(III) triflate to the same reaction vessel. To prove the synthetic potential of the resulting Michael adducts, one of the adducts was transformed into a pharmaceutically interesting dihydropyridazinone derivative.
中文翻译:

由反式-2-芳基-3-硝基环丙烷-1,1-二羧酸盐和活性芳烃催化三氟化硼促进的三氟甲磺酸铟(III)三氟甲磺酸酯催化的一锅法合成(1,2-二芳基-2-氧代乙基)丙二酸酯
描述了芳基亚甲基丙二酸酯与活化的芳族化合物的迈克尔加成物的顺序一锅合成。该方法包括治疗的反式-2-芳基-3-硝基-环丙烷-1,1-二羧酸酯与三氟化硼醚以形成aroylmethylidenemalonates原位再活化除了芳族化合物如吲哚,咔唑,吡咯,噻吩,和methoxybenzenes的苯并二氧杂环戊然后将催化量的三氟甲磺酸铟(III)输送到同一反应容器中。为了证明所得迈克尔加合物的合成潜力,将其中一种加合物转化为药学上感兴趣的二氢哒嗪酮衍生物。
更新日期:2015-06-10
中文翻译:

由反式-2-芳基-3-硝基环丙烷-1,1-二羧酸盐和活性芳烃催化三氟化硼促进的三氟甲磺酸铟(III)三氟甲磺酸酯催化的一锅法合成(1,2-二芳基-2-氧代乙基)丙二酸酯
描述了芳基亚甲基丙二酸酯与活化的芳族化合物的迈克尔加成物的顺序一锅合成。该方法包括治疗的反式-2-芳基-3-硝基-环丙烷-1,1-二羧酸酯与三氟化硼醚以形成aroylmethylidenemalonates原位再活化除了芳族化合物如吲哚,咔唑,吡咯,噻吩,和methoxybenzenes的苯并二氧杂环戊然后将催化量的三氟甲磺酸铟(III)输送到同一反应容器中。为了证明所得迈克尔加合物的合成潜力,将其中一种加合物转化为药学上感兴趣的二氢哒嗪酮衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号