当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical and Scalable Two-Step Process for 6-(2-Fluoro-4-nitrophenyl)-2-oxa-6-azaspiro[3.3]heptane: A Key Intermediate of the Potent Antibiotic Drug Candidate TBI-223
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-07-12 , DOI: 10.1021/acs.oprd.3c00148 Flavio S P Cardoso 1 , Appasaheb L Kadam 1 , Ryan C Nelson 1 , John W Tomlin 1 , Dipendra Dahal 2 , Christopher S Kuehner 2 , Gard Gudvangen 2 , Anthony J Arduengo 2 , Justina M Burns 1 , Sarah L Aleshire 1 , David R Snead 1 , Fengrui Qu 3 , Ken Belmore 3 , Saeed Ahmad 1 , Toolika Agrawal 4 , Joshua D Sieber 4 , Kai Oliver Donsbach 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-07-12 , DOI: 10.1021/acs.oprd.3c00148 Flavio S P Cardoso 1 , Appasaheb L Kadam 1 , Ryan C Nelson 1 , John W Tomlin 1 , Dipendra Dahal 2 , Christopher S Kuehner 2 , Gard Gudvangen 2 , Anthony J Arduengo 2 , Justina M Burns 1 , Sarah L Aleshire 1 , David R Snead 1 , Fengrui Qu 3 , Ken Belmore 3 , Saeed Ahmad 1 , Toolika Agrawal 4 , Joshua D Sieber 4 , Kai Oliver Donsbach 1
Affiliation
|
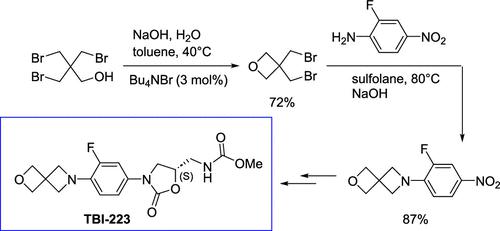
A low-cost, protecting group-free route to 6-(2-fluoro-4-nitrophenyl)-2-oxa-6-azaspiro[3.3]heptane (1), the starting material for the in-development tuberculosis treatment TBI-223, is described. The key bond forming step in this route is the creation of the azetidine ring through a hydroxide-facilitated alkylation of 2-fluoro-4-nitroaniline (2) with 3,3-bis(bromomethyl)oxetane (BBMO, 3). After optimization, this ring formation reaction was demonstrated at 100 g scale with isolated yield of 87% and final product purity of >99%. The alkylating agent 3 was synthesized using an optimized procedure that starts from tribromoneopentyl alcohol (TBNPA, 4), a commercially available flame retardant. Treatment of 4 with sodium hydroxide under Schotten–Baumann conditions closed the oxetane ring, and after distillation, 3 was recovered in 72% yield and >95% purity. This new approach to compound 1 avoids the previous drawbacks associated with the synthesis of 2-oxa-6-azaspiro[3,3]heptane (5), the major cost driver used in previous routes to TBI-223. The optimization and multigram scale-up results for this new route are reported herein.
中文翻译:

6-(2-氟-4-硝基苯基)-2-氧杂-6-氮杂螺[3.3]庚烷的实用且可扩展的两步法:强效抗生素候选药物 TBI-223 的关键中间体
一种低成本、无保护基的途径获得 6-(2-氟-4-硝基苯基)-2-氧杂-6-氮杂螺[3.3]庚烷 (1),这是正在开发的结核病治疗 TBI 的起始材料223,已描述。该路线中关键的成键步骤是通过 2-氟-4-硝基苯胺 ( 2 ) 与 3,3-双(溴甲基)氧杂环丁烷 (BBMO, 3 ) 的氢氧化物促进烷基化形成氮杂环丁烷环。经过优化,该成环反应在 100 g 规模下得到验证,分离收率为 87%,最终产品纯度 >99%。烷基化剂3是使用优化程序合成的,该程序以三溴新戊醇(TBNPA,4)(一种市售阻燃剂)为原料。在 Schotten-Baumann 条件下用氢氧化钠处理4 ,封闭了氧杂环丁烷环,蒸馏后,以 72% 的收率和 >95% 的纯度回收了3 。这种化合物1的新方法避免了先前与 2-oxa-6-azaspiro[3,3]heptane ( 5 )合成相关的缺点,2-oxa-6-azaspiro[3,3]heptane ( 5 ) 是先前 TBI-223 路线中使用的主要成本驱动因素。本文报告了该新路线的优化和多克放大结果。
更新日期:2023-07-12
中文翻译:

6-(2-氟-4-硝基苯基)-2-氧杂-6-氮杂螺[3.3]庚烷的实用且可扩展的两步法:强效抗生素候选药物 TBI-223 的关键中间体
一种低成本、无保护基的途径获得 6-(2-氟-4-硝基苯基)-2-氧杂-6-氮杂螺[3.3]庚烷 (1),这是正在开发的结核病治疗 TBI 的起始材料223,已描述。该路线中关键的成键步骤是通过 2-氟-4-硝基苯胺 ( 2 ) 与 3,3-双(溴甲基)氧杂环丁烷 (BBMO, 3 ) 的氢氧化物促进烷基化形成氮杂环丁烷环。经过优化,该成环反应在 100 g 规模下得到验证,分离收率为 87%,最终产品纯度 >99%。烷基化剂3是使用优化程序合成的,该程序以三溴新戊醇(TBNPA,4)(一种市售阻燃剂)为原料。在 Schotten-Baumann 条件下用氢氧化钠处理4 ,封闭了氧杂环丁烷环,蒸馏后,以 72% 的收率和 >95% 的纯度回收了3 。这种化合物1的新方法避免了先前与 2-oxa-6-azaspiro[3,3]heptane ( 5 )合成相关的缺点,2-oxa-6-azaspiro[3,3]heptane ( 5 ) 是先前 TBI-223 路线中使用的主要成本驱动因素。本文报告了该新路线的优化和多克放大结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号