Phytochemistry Letters ( IF 1.3 ) Pub Date : 2023-07-11 , DOI: 10.1016/j.phytol.2023.07.009 Jinmeng Yu , Ming Gao , Rongmei Zhao , Haifeng Liu , Hailing Fan , Le Pan , Lu Jin
|
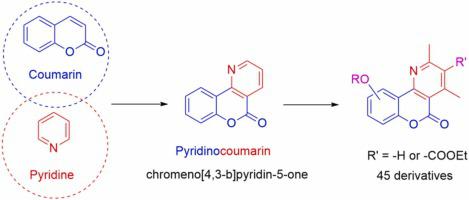
Based on the scaffold of chromeno[4,3-b]pyridin-5-one found in a natural product (polynemoraline C), a series of 45 novel derivatives have been designed and investigated for their fungicide activity and corresponding synthesis methods. All compounds were characterized by 1H NMR and 13C NMR, and some by HR-MS. The antifungal activities were screened at 100 µg/mL against Alternaria alternata, Alternaria solani, Botrytis cinerea, Fusarium oxysporum. The results showed that most of the compounds were more effective against Alternaria alternata and Alternaria solani than Botrytis cinerea and Fusarium oxysporum. Some compounds were better than the positive controls. Among the synthesized derivatives, compound 1b showed remarkable activity against Alternaria alternata and Alternaria solani. The inhibition rate of compound 1b against Alternaria alternata at 100 µg/mL reached 62.09 %, which was higher than those of the positive controls, chlorothaloni (38.81 %) and azoxystrobin (50.06 %), while it was 54.61 % against Alternaria solani, which was higher than that of chlorothaloni (44.67 %) and almost equal to azoxystrobin (57.43 %). The EC50 values of compound 1b for Alternaria alternata and Alternaria solani were 62.73 and 65.95 μg/mL respectively. Structurally, the side chains of specific ester groups attached to the C-7 site of some compounds exhibit excellent inhibitory activity. The structure-activity relationship suggested that the length of the four carbon atoms in the side chain had significant effects on the antifungal activity. Our results revealed that a new series of chromeno[4,3-b]pyridin-5-one showed potential as an effective antifungal scaffold, and also provided a possibility for the structure design optimization, synthesis and development of a novel fungicide based on natural products.
中文翻译:

色并[4,3-b]吡啶-5-酮衍生物的设计、合成及抗真菌活性评价
基于天然产物(polynemoraline C)中发现的色烯基[4,3- b ]吡啶-5-酮支架,设计并研究了一系列45种新型衍生物的杀菌活性和相应的合成方法。所有化合物均通过1 H NMR 和13 C NMR进行表征,部分化合物通过 HR-MS 进行表征。筛选了 100 µg/mL 浓度下对链格孢、茄链格孢、灰葡萄孢、尖孢镰刀菌的抗真菌活性。结果表明,大多数化合物对Alternaria alternata和Alternaria solani的作用比对Botrytis cinerea更有效。和尖镰孢。一些化合物优于阳性对照。在合成的衍生物中,化合物1b对Alternaria alternata和Alternaria solani表现出显着的活性。化合物1b在100 µg/mL浓度下对链格孢菌的抑制率达到62.09 %,高于阳性对照百菌清(38.81 %)和嘧菌酯(50.06 %),而对茄斑病菌的抑制率为54.61 %,显着高于阳性对照百菌清( 38.81 %)和嘧菌酯(50.06 %)。高于百菌清(44.67%),与嘧菌酯(57.43%)基本持平。化合物1b对于Alternaria alternata和Alternaria solani的EC 50值分别为 62.73 和 65.95 μg/mL。从结构上看,一些化合物的C-7位点上连接的特定酯基侧链表现出优异的抑制活性。构效关系表明侧链四个碳原子的长度对其抗真菌活性有显着影响。我们的研究结果表明,一系列新的色并[4,3- b ]pyridin-5-one显示出作为有效抗真菌支架的潜力,也为基于天然产物的新型杀菌剂的结构设计优化、合成和开发提供了可能性。产品。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号