当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Planar-Chiral Oxazole–Pyridine N,N-Ligands: Application in Palladium-Catalyzed Asymmetric Acetoxylative Cyclization
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-07-11 , DOI: 10.1021/acscatal.3c01163 Yu-Qing Bai 1, 2 , Xin-Wei Wang 1 , Bo Wu 1 , Xiao-Qing Wang 1 , Rong-Zheng Liao 3 , Man Li 3 , Yong-Gui Zhou 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-07-11 , DOI: 10.1021/acscatal.3c01163 Yu-Qing Bai 1, 2 , Xin-Wei Wang 1 , Bo Wu 1 , Xiao-Qing Wang 1 , Rong-Zheng Liao 3 , Man Li 3 , Yong-Gui Zhou 1
Affiliation
|
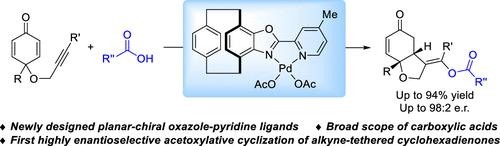
The development of chiral ligands to fine-tune the stereocontrol has been recognized as a crucial pillar of asymmetric catalysis. In contrast to the well-developed chiral pyridine–pyridine-type and pyridine–oxazoline-type ligands, chiral oxazole–pyridine-type ligands have rarely been exploited. In this study, a class of [2.2]paracyclophane-based planar-chiral oxazole–pyridine N,N-ligands have been designed and synthesized. These ligands presented a superior performance in the enantioselective palladium-catalyzed asymmetric acetoxylative cyclization of alkyne-tethered cyclohexadienones, providing the chiral cis-hydrobenzofurans that belong to bioactive molecules with potent NF-κB inhibition in broad substrate scope. These results demonstrated the promising potential of the chiral oxazole–pyridine ligands as an efficient type of N,N-ligand scaffold.
中文翻译:

平面手性恶唑-吡啶 N,N-配体的设计与合成:在钯催化不对称乙酰氧基化环化中的应用
开发手性配体来微调立体控制已被认为是不对称催化的关键支柱。与成熟的手性吡啶-吡啶型和吡啶-恶唑啉型配体相比,手性恶唑-吡啶型配体很少被开发。在本研究中,设计并合成了一类基于[2.2]对环芳烷的平面手性恶唑-吡啶N , N-配体。这些配体在对映选择性钯催化的炔系环己二酮的不对称乙酰氧基化环化中表现出优异的性能,提供手性顺式-氢苯并呋喃属于生物活性分子,在广泛的底物范围内具有有效的 NF-κB 抑制作用。这些结果证明了手性恶唑-吡啶配体作为一种有效的N , N -配体支架的巨大潜力。
更新日期:2023-07-11
中文翻译:

平面手性恶唑-吡啶 N,N-配体的设计与合成:在钯催化不对称乙酰氧基化环化中的应用
开发手性配体来微调立体控制已被认为是不对称催化的关键支柱。与成熟的手性吡啶-吡啶型和吡啶-恶唑啉型配体相比,手性恶唑-吡啶型配体很少被开发。在本研究中,设计并合成了一类基于[2.2]对环芳烷的平面手性恶唑-吡啶N , N-配体。这些配体在对映选择性钯催化的炔系环己二酮的不对称乙酰氧基化环化中表现出优异的性能,提供手性顺式-氢苯并呋喃属于生物活性分子,在广泛的底物范围内具有有效的 NF-κB 抑制作用。这些结果证明了手性恶唑-吡啶配体作为一种有效的N , N -配体支架的巨大潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号