Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-07-10 , DOI: 10.1016/j.cej.2023.144652 Young-Hwan Jo , Won-Gune Jeong , Jin Park , Kitae Baek
|
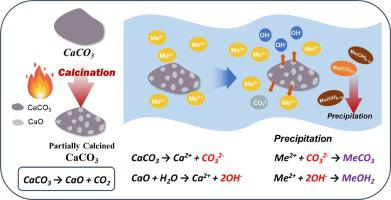
Calcium-based materials, known for their ease of handling and cost-effectiveness, are commonly used for removing heavy metals from groundwater. Unfortunately, among the calcium-based materials, calcium carbonate (CaCO3) is not a viable means of removing heavy metals due to its low solubility. Calcium oxide (CaO) with high solubility, obtained through the calcination of CaCO3, does effectively remove most heavy metals by raising the pH to alkaline condition. However, the dissolution of CaO increases the system temperature rapidly due to the heat associated with hydration, and excessive alkalinity is released due to rapid dissolution and pores become clogged due to hardening. To overcome these problems, this study proposed partially calcined CaCO3 (PCC) as a potential remediator of groundwater contaminated with multi-heavy metals. Scanning electron microscopy (SEM) images and X-ray diffraction (XRD) analysis confirmed the co-existence of CaCO3 and CaO phases in one material. In the partially calcined CaCO3 samples, the amount of CaO present increased with calcination temperature and residence time. More CaO was observed to result in greater removal of heavy metals. Compared to a binary mixture of CaO and CaCO3, PCC showed a slower dissolution rate of CaO, indicating control over CaO dissolution kinetics. It was determined that 1 kg of PCC was capable of purifying approximately 154.6 L of contaminated groundwater with an 18.2 wt.% CaO content, twice as much as the binary mixture of CaO and CaCO3. Based on the results, partially calcined CaCO3 is a potential reactive medium to remediate groundwater contaminated with heavy metals.
中文翻译:

部分煅烧碳酸钙修复多种重金属污染地下水
钙基材料以其易于处理和成本效益而闻名,通常用于去除地下水中的重金属。不幸的是,在钙基材料中,碳酸钙(CaCO 3 )由于其溶解度低而不是去除重金属的可行方法。通过CaCO 3的煅烧获得的高溶解度氧化钙(CaO)可以通过将pH值提高到碱性条件来有效去除大多数重金属。然而,由于水化产生的热量,CaO 的溶解使系统温度迅速升高,并且由于快速溶解而释放过量的碱度,并且由于硬化而堵塞孔隙。为了克服这些问题,本研究提出部分煅烧的CaCO 3(PCC)作为受多种重金属污染的地下水的潜在修复剂。扫描电子显微镜(SEM)图像和X射线衍射(XRD)分析证实了CaCO 3和CaO相在一种材料中共存。在部分煅烧的CaCO 3样品中,CaO 的含量随着煅烧温度和停留时间的增加而增加。观察到更多的 CaO 会导致更多的重金属去除。与 CaO 和 CaCO 3的二元混合物相比,PCC 显示出较慢的 CaO 溶解速率,表明对 CaO 溶解动力学的控制。经测定,1 kg PCC 能够净化约 154.6 L 受污染的地下水,其中 CaO 含量为 18.2 wt.%,是 CaO 和 CaCO 二元混合物的两倍3 . 根据结果,部分煅烧的CaCO 3是修复受重金属污染的地下水的潜在反应介质。





























 京公网安备 11010802027423号
京公网安备 11010802027423号