当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,5,7-Triazabicyclo[4.4.0]dec-5-ene: An Effective Catalyst for Amide Formation by Lactone Aminolysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-07-11 , DOI: 10.1021/acs.joc.3c00913 Chunling Blue Lan 1 , Karine Auclair 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-07-11 , DOI: 10.1021/acs.joc.3c00913 Chunling Blue Lan 1 , Karine Auclair 1
Affiliation
|
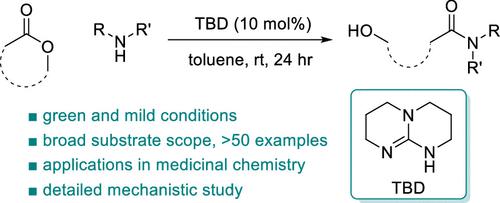
The amide is one of the most prevalent functional groups throughout natural and engineered chemical space. Among various methods of constructing amide bonds, lactone aminolysis remains one of the most atom economical. Herein, we report 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) as an effective catalyst for lactone aminolysis under mild conditions. This methodology is compatible with a wide range of lactones and amines (>50 examples), including various natural products and pharmaceuticals, and applicable to the synthesis of bioactive molecules. Detailed mechanistic studies under synthetically relevant conditions, including reaction progress kinetic analysis and variable time normalization analysis, reveal a likely mechanism for this reaction involving acyl-TBD as the reactive intermediate.
中文翻译:

1,5,7-三氮杂双环[4.4.0]dec-5-ene:内酯氨解形成酰胺的有效催化剂
酰胺是天然和工程化学领域最普遍的官能团之一。在构建酰胺键的各种方法中,内酯氨解仍然是最经济的原子经济方法之一。在此,我们报道了 1,5,7-三氮杂双环[4.4.0]dec-5-ene (TBD) 作为温和条件下内酯氨解的有效催化剂。该方法与多种内酯和胺(> 50 个示例)兼容,包括各种天然产物和药物,并且适用于生物活性分子的合成。在综合相关条件下的详细机理研究,包括反应进程动力学分析和可变时间归一化分析,揭示了涉及酰基-TBD作为反应中间体的该反应的可能机制。
更新日期:2023-07-11
中文翻译:

1,5,7-三氮杂双环[4.4.0]dec-5-ene:内酯氨解形成酰胺的有效催化剂
酰胺是天然和工程化学领域最普遍的官能团之一。在构建酰胺键的各种方法中,内酯氨解仍然是最经济的原子经济方法之一。在此,我们报道了 1,5,7-三氮杂双环[4.4.0]dec-5-ene (TBD) 作为温和条件下内酯氨解的有效催化剂。该方法与多种内酯和胺(> 50 个示例)兼容,包括各种天然产物和药物,并且适用于生物活性分子的合成。在综合相关条件下的详细机理研究,包括反应进程动力学分析和可变时间归一化分析,揭示了涉及酰基-TBD作为反应中间体的该反应的可能机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号