当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Formation of Hydroxyacetone, Acetone, and 1,2-Propanediol from Electrochemical CO2 Reduction on Copper
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-10 , DOI: 10.1021/jacs.3c03045
Alisson H M da Silva 1 , Georgios Karaiskakis 1 , Rafaël E Vos 1 , Marc T M Koper 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-10 , DOI: 10.1021/jacs.3c03045
Alisson H M da Silva 1 , Georgios Karaiskakis 1 , Rafaël E Vos 1 , Marc T M Koper 1
Affiliation
|
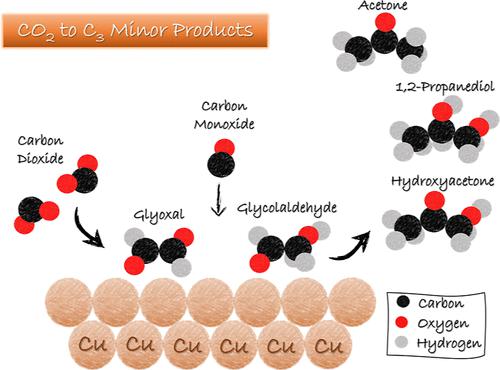
Studies focused on the mechanism of CO2 electroreduction (CO2RR) aim to open up opportunities to optimize reaction parameters toward selective synthesis of desired products. However, the reaction pathways for C3 compound syntheses, especially for minor compounds, remain incompletely understood. In this study, we investigated the formation pathway for hydroxyacetone, acetone, and 1,2-propanediol through CO(2)RR, which are minor products that required long electrolysis times to be detected. Our proposed reaction mechanism is based on a systematic investigation of the reduction of several functional groups on a Cu electrode, including aldehydes, ketones, ketonealdehydes, hydroxyls, hydroxycarbonyls, and hydroxydicarbonyls, as well as the coupling between CO and C2-dicarbonyl (glyoxal) or C2-hydroxycarbonyl (glycolaldehyde). This study allowed us to derive the fundamental principles of the reduction of functional groups on Cu electrodes. Our findings suggest that the formation of ethanol does not follow the glyoxal pathway, as previously suggested but instead likely occurs via the coupling of CH3* and CO. For the C3 compounds, our results suggest that 1,2-propanediol and acetone follow the hydroxyacetone pathway during CO2RR. Hydroxyacetone is likely formed through the coupling of CO and a C2-hydroxycarbonyl intermediate, such as a glycolaldehyde-like compound, as confirmed by adding glycolaldehyde to the CO(2)-saturated solution. This finding is consistent with CO2RR product distribution, as glycolaldehyde formation during CO2RR is limited, which, in turn, limits hydroxyacetone production. Our study contributes to a better understanding of the reaction mechanism for hydroxyacetone, acetone, and 1,2-propanediol synthesis from CO2RR and gives insights into these interesting compounds that may be formed electrochemically.
中文翻译:

铜上电化学 CO2 还原形成羟基丙酮、丙酮和 1,2-丙二醇的机理见解
重点研究CO 2电还原(CO 2 RR)机制,旨在为优化反应参数以选择性合成所需产物提供机会。然而,C 3化合物合成的反应途径,尤其是次要化合物的反应途径,仍不完全清楚。在本研究中,我们研究了羟基丙酮、丙酮和 1,2-丙二醇通过 CO (2) RR 的形成途径,这些都是需要较长电解时间才能检测到的次要产物。我们提出的反应机理是基于对 Cu 电极上几个官能团的还原的系统研究,包括醛、酮、酮醛、羟基、羟基羰基和羟基二羰基,以及 CO 和 C 2 -二羰基(乙二醛)之间的偶联。 )或C 2 -羟基羰基(乙醇醛)。这项研究使我们得出了铜电极上官能团还原的基本原理。我们的研究结果表明,乙醇的形成并不遵循乙二醛途径,如之前所建议的,而是可能通过 CH 3 * 和 CO的偶联发生。对于 C 3化合物,我们的结果表明 1,2-丙二醇和丙酮遵循CO 2 RR过程中的羟基丙酮途径。羟基丙酮可能是通过CO和C 2 -羟基羰基中间体(例如乙醇醛类化合物)的偶联形成的,这通过将乙醇醛添加到CO (2) -饱和溶液中来证实。这一发现与CO 2 RR产物分布一致,因为CO 2 RR过程中乙醇醛的形成受到限制,这反过来又限制了羟基丙酮的产生。我们的研究有助于更好地理解从 CO 2 RR 合成羟基丙酮、丙酮和 1,2-丙二醇的反应机制,并深入了解这些可能通过电化学形成的有趣化合物。
更新日期:2023-07-10
中文翻译:

铜上电化学 CO2 还原形成羟基丙酮、丙酮和 1,2-丙二醇的机理见解
重点研究CO 2电还原(CO 2 RR)机制,旨在为优化反应参数以选择性合成所需产物提供机会。然而,C 3化合物合成的反应途径,尤其是次要化合物的反应途径,仍不完全清楚。在本研究中,我们研究了羟基丙酮、丙酮和 1,2-丙二醇通过 CO (2) RR 的形成途径,这些都是需要较长电解时间才能检测到的次要产物。我们提出的反应机理是基于对 Cu 电极上几个官能团的还原的系统研究,包括醛、酮、酮醛、羟基、羟基羰基和羟基二羰基,以及 CO 和 C 2 -二羰基(乙二醛)之间的偶联。 )或C 2 -羟基羰基(乙醇醛)。这项研究使我们得出了铜电极上官能团还原的基本原理。我们的研究结果表明,乙醇的形成并不遵循乙二醛途径,如之前所建议的,而是可能通过 CH 3 * 和 CO的偶联发生。对于 C 3化合物,我们的结果表明 1,2-丙二醇和丙酮遵循CO 2 RR过程中的羟基丙酮途径。羟基丙酮可能是通过CO和C 2 -羟基羰基中间体(例如乙醇醛类化合物)的偶联形成的,这通过将乙醇醛添加到CO (2) -饱和溶液中来证实。这一发现与CO 2 RR产物分布一致,因为CO 2 RR过程中乙醇醛的形成受到限制,这反过来又限制了羟基丙酮的产生。我们的研究有助于更好地理解从 CO 2 RR 合成羟基丙酮、丙酮和 1,2-丙二醇的反应机制,并深入了解这些可能通过电化学形成的有趣化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号