当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cathode Kinetics Evaluation in Lean-Electrolyte Lithium–Sulfur Batteries
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-10 , DOI: 10.1021/jacs.3c02786 Zi-Xian Chen 1, 2 , Qian Cheng 1, 2 , Xi-Yao Li 3 , Zheng Li 3 , Yun-Wei Song 3 , Furong Sun 3, 4 , Meng Zhao 1, 2 , Xue-Qiang Zhang 1, 2 , Bo-Quan Li 1, 2 , Jia-Qi Huang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-10 , DOI: 10.1021/jacs.3c02786 Zi-Xian Chen 1, 2 , Qian Cheng 1, 2 , Xi-Yao Li 3 , Zheng Li 3 , Yun-Wei Song 3 , Furong Sun 3, 4 , Meng Zhao 1, 2 , Xue-Qiang Zhang 1, 2 , Bo-Quan Li 1, 2 , Jia-Qi Huang 1, 2
Affiliation
|
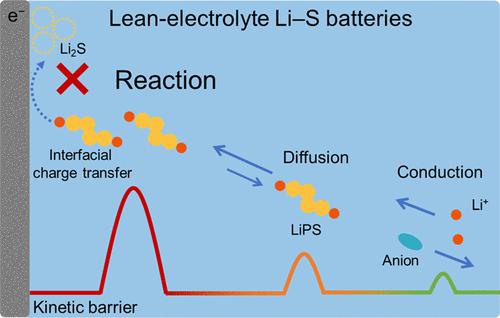
Lithium–sulfur (Li–S) batteries afford great promise on achieving practical high energy density beyond lithium-ion batteries. Lean-electrolyte conditions constitute the prerequisite for achieving high-energy-density Li–S batteries but inevitably deteriorates battery performances, especially the sulfur cathode kinetics. Herein, the polarizations of the sulfur cathode are systematically decoupled to identify the key kinetic limiting factor in lean-electrolyte Li–S batteries. Concretely, an electrochemical impedance spectroscopy combined galvanostatic intermittent titration technique method is developed to decouple the cathodic polarizations into activation, concentration, and ohmic parts. Therein, activation polarization during lithium sulfide nucleation emerges as the dominant polarization as the electrolyte-to-sulfur ratio (E/S ratio) decreases, and the sluggish interfacial charge transfer kinetics is identified as the main reason for degraded cell performances under lean-electrolyte conditions. Accordingly, a lithium bis(fluorosulfonyl)imide electrolyte is proposed to decrease activation polarization, and Li–S batteries adopting this electrolyte provide a discharge capacity of 985 mAh g–1 under a low E/S ratio of 4 μL mg–1 at 0.2 C. This work identifies the key kinetic limiting factor of lean-electrolyte Li–S batteries and provides guidance on designing rational promotion strategies to achieve advanced Li–S batteries.
中文翻译:

贫电解质锂硫电池的阴极动力学评估
锂硫(Li-S)电池在实现超越锂离子电池的实用高能量密度方面具有广阔的前景。贫电解质条件是实现高能量密度锂硫电池的先决条件,但不可避免地会恶化电池性能,尤其是硫正极动力学。在这里,硫阴极的极化被系统地解耦,以确定贫电解质锂硫电池中的关键动力学限制因素。具体而言,开发了电化学阻抗谱结合恒电流间歇滴定技术方法,将阴极极化解耦为活化、浓度和欧姆部分。其中,随着电解质与硫的比(E/S比)降低,硫化锂成核过程中的活化极化成为主要极化,并且缓慢的界面电荷转移动力学被认为是贫电解质下电池性能下降的主要原因状况。因此,提出了双(氟磺酰基)亚胺锂电解质来降低活化极化,采用该电解质的Li-S电池在0.2时在4 μL mg –1的低E/S比下提供985 mAh g –1的放电容量C. 这项工作确定了贫电解质锂硫电池的关键动力学限制因素,并为设计合理的推广策略以实现先进的锂硫电池提供了指导。
更新日期:2023-07-10
中文翻译:

贫电解质锂硫电池的阴极动力学评估
锂硫(Li-S)电池在实现超越锂离子电池的实用高能量密度方面具有广阔的前景。贫电解质条件是实现高能量密度锂硫电池的先决条件,但不可避免地会恶化电池性能,尤其是硫正极动力学。在这里,硫阴极的极化被系统地解耦,以确定贫电解质锂硫电池中的关键动力学限制因素。具体而言,开发了电化学阻抗谱结合恒电流间歇滴定技术方法,将阴极极化解耦为活化、浓度和欧姆部分。其中,随着电解质与硫的比(E/S比)降低,硫化锂成核过程中的活化极化成为主要极化,并且缓慢的界面电荷转移动力学被认为是贫电解质下电池性能下降的主要原因状况。因此,提出了双(氟磺酰基)亚胺锂电解质来降低活化极化,采用该电解质的Li-S电池在0.2时在4 μL mg –1的低E/S比下提供985 mAh g –1的放电容量C. 这项工作确定了贫电解质锂硫电池的关键动力学限制因素,并为设计合理的推广策略以实现先进的锂硫电池提供了指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号