当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium(III)-Catalyzed C–H Cyclization of Sulfoximines with Diazo Meldrum’s Acids for the Synthesis of Cyclic Sulfoximines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-07-10 , DOI: 10.1021/acs.joc.3c00984 Gi Uk Han 1 , Suhui Kim 1 , Sang Hoon Han 1 , Chanyoung Maeng 1 , Gi Hoon Ko 1 , Kyungsup Lee 1 , Hee Chan Noh 1 , Phil Ho Lee 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-07-10 , DOI: 10.1021/acs.joc.3c00984 Gi Uk Han 1 , Suhui Kim 1 , Sang Hoon Han 1 , Chanyoung Maeng 1 , Gi Hoon Ko 1 , Kyungsup Lee 1 , Hee Chan Noh 1 , Phil Ho Lee 1
Affiliation
|
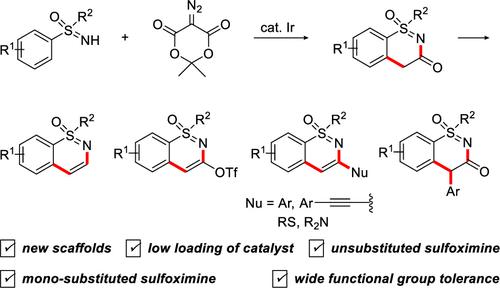
Iridium(III)-catalyzed C–H cyclization of sulfoximines with diazo Meldrum’s acid provided cyclic sulfoximines with a carbonyl group in good to excellent yields. These compounds were easily converted into unsubstituted and arylated sulfoximines. Moreover, the vinyl triflates obtained from the cyclic sulfoximines underwent palladium(II)-catalyzed cross-coupling reactions with a variety of aryl, arylalkynyl, and heteroatom (N and S) nucleophiles, affording a wide range of monosubstituted sulfoximines in high yields.
中文翻译:

铱(III)催化亚磺酰亚胺与重氮Meldrum酸的C-H环化反应合成环状亚磺酰亚胺
铱 (III) 催化的亚砜亚胺与重氮 Meldrum 酸的 C-H 环化反应以良好至优异的收率提供了带有羰基的环状亚砜亚胺。这些化合物很容易转化为未取代的和芳基化的亚砜亚胺。此外,由环状亚砜亚胺获得的乙烯基三氟甲磺酸酯与各种芳基、芳基炔基和杂原子(N和S)亲核试剂进行钯(II)催化的交叉偶联反应,以高产率提供各种单取代亚砜亚胺。
更新日期:2023-07-10
中文翻译:

铱(III)催化亚磺酰亚胺与重氮Meldrum酸的C-H环化反应合成环状亚磺酰亚胺
铱 (III) 催化的亚砜亚胺与重氮 Meldrum 酸的 C-H 环化反应以良好至优异的收率提供了带有羰基的环状亚砜亚胺。这些化合物很容易转化为未取代的和芳基化的亚砜亚胺。此外,由环状亚砜亚胺获得的乙烯基三氟甲磺酸酯与各种芳基、芳基炔基和杂原子(N和S)亲核试剂进行钯(II)催化的交叉偶联反应,以高产率提供各种单取代亚砜亚胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号