Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-07-08 , DOI: 10.1016/j.bioorg.2023.106727 Kun Liu 1 , Min Mo 1 , Gang Yu 1 , Jia Yu 1 , Shan-Min Song 2 , Sha Cheng 1 , Hui-Min Li 1 , Xue-Ling Meng 1 , Xiao-Ping Zeng 1 , Guang-Can Xu 1 , Heng Luo 1 , Bi-Xue Xu 1
|
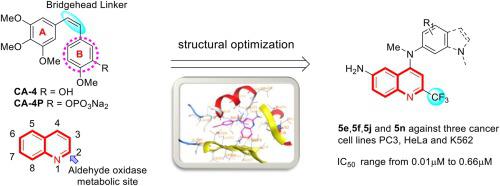
In this work, a series of 2-(trifluoromethyl)quinolin-4-amine derivatives were designed and synthesized through structural optimization strategy as a microtubule-targeted agents (MTAs) and their cytotoxicity activity against PC3, K562 and HeLa cell lines were evaluated. The half maximal inhibitory concentration (IC50) of 5e, 5f, and 5o suggested that their potency of anti-proliferative activities against HeLa cell lines were better than the combretastatin A-4. Compound 5e showed the higher anti-proliferative activity against PC3, K562 and HeLa in vitro with IC50 values of 0.49 µM, 0.08 µM and 0.01 µM, respectively. Further mechanism study indicated that the representative compound 5e was new class of tubulin inhibitors by EBI competition assay and tubulin polymerization assays, it is similar to colchicine. Immunofluorescence staining revealed that compound 5e apparently disrupted tubulin network in HeLa cells, and compound 5e arrested HeLa cells at the G2/M phase and induced cells apoptosis in a dose-dependent manner. Molecular docking results illustrated that the hydrogen bonds of represented compounds reinforced the interactions in the pocket of colchicine binding site. Preliminary results suggested that 5e deserves further research as a promising tubulin inhibitor for the development of anticancer agents.
中文翻译:

发现新型 2-(三氟甲基)喹啉-4-胺衍生物作为具有微管聚合抑制活性的有效抗肿瘤剂
本工作通过结构优化策略设计并合成了一系列2-(三氟甲基)喹啉-4-胺衍生物作为微管靶向药物(MTA),并评估了它们对PC3、K562和HeLa细胞系的细胞毒活性。5e、5f和5o的半数抑制浓度(IC 50 )表明它们对HeLa细胞系的抗增殖活性优于考布他汀A-4。化合物5e在体外对PC3、K562和HeLa表现出较高的抗增殖活性,IC 50值分别为0.49 µM、0.08 µM和0.01 µM。进一步的机理研究表明,通过EBI竞争试验和微管蛋白聚合试验,代表化合物5e是一类新型微管蛋白抑制剂,与秋水仙碱相似。免疫荧光染色显示,化合物5e明显破坏了HeLa细胞中的微管蛋白网络,并且化合物5e以剂量依赖性方式将HeLa细胞阻滞在G2/M期并诱导细胞凋亡。分子对接结果表明,代表化合物的氢键增强了秋水仙碱结合位点口袋中的相互作用。初步结果表明,5e作为一种有前景的微管蛋白抑制剂,值得进一步研究,用于抗癌药物的开发。






























 京公网安备 11010802027423号
京公网安备 11010802027423号