Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2023-07-08 , DOI: 10.1016/j.saa.2023.123115
Yan-Jun Li 1 , Cai-Cai Liang 1 , Ling Jin 2 , Juan Chen 1
|
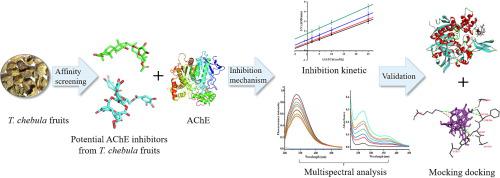
Acetylcholinesterase (AChE) is an important therapeutic target for the treatment of Alzheimer's disease (AD), and the development of natural AChE inhibitors as candidates has played a significant role in drug discovery. In this study, the inhibition mechanisms of four ellagitannins, punicalagin, chebulinic acid, geraniin and corilagin, from Terminalia chebula fruits on AChE were investigated systematically by a combination of inhibition kinetics, multi-spectroscopic methods and molecular docking. The kinetic results showed that punicalagin, chebulinic acid and geraniin exhibited strong reversible inhibitory effects on AChE in an uncompetitive manner with the IC50 values of 0.43, 0.50, and 0.51 mM, respectively, while corilagin inhibited AChE activity in a mixed type with the IC50 value of 0.72 mM. The results of fluorescence and UV-vis spectra and fluorescence resonance energy transfer (FRET) revealed that four ellagitannins could significantly quenched the intrinsic fluorescence of AChE though a static quenching along with non-radiative energy transfer. Thermodynamic analyses showed that values of ΔG, ΔH and ΔS were negative, indicating that all binding processes were spontaneous, and the hydrogen bonding and Van der Waals forces might make a great contribution to the formation of inhibitor-AChE complexes. The synchronous fluorescence, three-dimensional (3D) fluorescence, UV-vis, and FT-IR spectra studies suggested that four ellagitannins could lead to alterations in the micro-environment and secondary structure of AChE, and thus the conformational change of AChE. Moreover, molecular docking demonstrated that four ellagitannins could interacted with main amino acid residues of AChE with affinity energies ranging from -9.9 to -8.7 kJ/mol, and further confirmed the above experimental results. This study provided valuable findings for the potential application of four ellagitannins as promising candidates in the exploration of natural AChE inhibitors for the treatment of AD.
中文翻译:

通过抑制动力学、光谱学和分子对接分析研究榄仁果实中四种鞣花单宁对乙酰胆碱酯酶的抑制机制
乙酰胆碱酯酶(AChE)是治疗阿尔茨海默病(AD)的重要治疗靶点,天然AChE抑制剂的开发作为候选药物在药物发现中发挥了重要作用。本研究采用抑制动力学、多光谱方法和分子对接相结合的方法,系统研究了榄仁果实中4种鞣花单宁、安石榴苷、车布林酸、香叶素和柯里拉苷对AChE的抑制机制。动力学结果表明,安石榴苷、车布林酸和香叶苷以非竞争性方式对AChE表现出强烈的可逆抑制作用,IC 50值分别为0.43、0.50和0.51 mM,而柯里拉京与IC以混合型抑制AChE活性。50值为 0.72 mM。荧光光谱、紫外可见光谱和荧光共振能量转移(FRET)结果表明,四种鞣花单宁可以通过静态猝灭和非辐射能量转移显着猝灭AChE的固有荧光。热力学分析表明,ΔG、ΔH和ΔS均为负值,表明所有结合过程都是自发的,氢键和范德华力可能对抑制剂-AChE复合物的形成有很大贡献。同步荧光、三维(3D)荧光、紫外可见光谱和傅立叶变换红外光谱研究表明,四种鞣花单宁可导致AChE微环境和二级结构的改变,从而引起AChE的构象变化。此外,分子对接表明,4种鞣花单宁能够与AChE的主要氨基酸残基相互作用,亲和能范围为-9.9~-8.7 kJ/mol,进一步证实了上述实验结果。这项研究为四种鞣花单宁作为探索天然乙酰胆碱酯酶抑制剂治疗 AD 的有希望的候选者的潜在应用提供了有价值的发现。




































 京公网安备 11010802027423号
京公网安备 11010802027423号