当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the Electronic Structure and Dynamics of the Atomically Dispersed Iron Sites in Electrochemical CO2 Reduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-07 , DOI: 10.1021/jacs.3c05457 Yaqiong Zeng, Jian Zhao, Shifu Wang, Xinyi Ren, Yuanlong Tan, Ying-Rui Lu, Shibo Xi, Junhu Wang, Frédéric Jaouen, Xuning Li, Yanqiang Huang, Tao Zhang, Bin Liu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-07 , DOI: 10.1021/jacs.3c05457 Yaqiong Zeng, Jian Zhao, Shifu Wang, Xinyi Ren, Yuanlong Tan, Ying-Rui Lu, Shibo Xi, Junhu Wang, Frédéric Jaouen, Xuning Li, Yanqiang Huang, Tao Zhang, Bin Liu
|
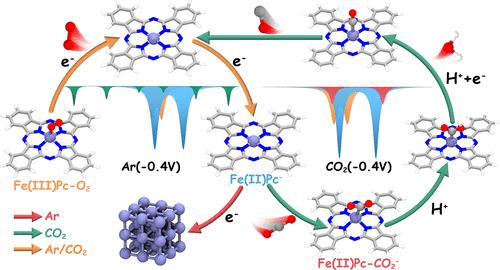
Single-atom catalysts with a well-defined metal center open unique opportunities for exploring the catalytically active site and reaction mechanism of chemical reactions. However, understanding of the electronic and structural dynamics of single-atom catalytic centers under reaction conditions is still limited due to the challenge of combining operando techniques that are sensitive to such sites and model single-atom systems. Herein, supported by state-of-the-art operando techniques, we provide an in-depth study of the dynamic structural and electronic evolution during the electrochemical CO2 reduction reaction (CO2RR) of a model catalyst comprising iron only as a high-spin (HS) Fe(III)N4 center in its resting state. Operando 57Fe Mössbauer and X-ray absorption spectroscopies clearly evidence the change from a HS Fe(III)N4 to a HS Fe(II)N4 center with decreasing potential, CO2- or Ar-saturation of the electrolyte, leading to different adsorbates and stability of the HS Fe(II)N4 center. With operando Raman spectroscopy and cyclic voltammetry, we identify that the phthalocyanine (Pc) ligand coordinating the iron cation center undergoes a redox process from Fe(II)Pc to Fe(II)Pc–. Altogether, the HS Fe(II)Pc– species is identified as the catalytic intermediate for CO2RR. Furthermore, theoretical calculations reveal that the electroreduction of the Pc ligand modifies the d-band center of the in situ generated HS Fe(II)Pc– species, resulting in an optimal binding strength to CO2 and thus boosting the catalytic performance of CO2RR. This work provides both experimental and theoretical evidence toward the electronic structural and dynamics of reactive sites in single-Fe-atom materials and shall guide the design of novel efficient catalysts for CO2RR.
中文翻译:

揭示电化学 CO2 还原中原子分散铁位点的电子结构和动力学
具有明确金属中心的单原子催化剂为探索化学反应的催化活性位点和反应机制提供了独特的机会。然而,由于将对此类位点敏感的操作技术与单原子系统模型相结合的挑战,对反应条件下单原子催化中心的电子和结构动力学的理解仍然有限。在此,在最先进的操作技术的支持下,我们对仅包含铁作为高浓度催化剂的模型催化剂的电化学 CO 2还原反应 (CO 2 RR)过程中的动态结构和电子演化进行了深入研究。-自旋 (HS) Fe(III)N 4中心处于静止状态。Operando 57 Fe 穆斯堡尔和 X 射线吸收光谱清楚地证明了从 HS Fe(III)N 4到 HS Fe(II)N 4中心的变化,并且电解质的电势、CO 2 - 或 Ar 饱和度降低,导致HS Fe(II)N 4中心的不同吸附物和稳定性。通过操作拉曼光谱和循环伏安法,我们发现与铁阳离子中心配位的酞菁(Pc)配体经历了从 Fe(II)Pc 到 Fe(II)Pc – 的氧化还原过程。总之,HS Fe(II)Pc –物质被确定为 CO 2 RR 的催化中间体。此外,理论计算表明,Pc配体的电还原改变了原位生成的HS Fe(II)Pc -物种的d带中心,导致与CO 2的最佳结合强度,从而提高了CO 2的催化性能RR。这项工作为单铁原子材料中反应位点的电子结构和动力学提供了实验和理论证据,并将指导新型高效CO 2 RR催化剂的设计。
更新日期:2023-07-07
中文翻译:

揭示电化学 CO2 还原中原子分散铁位点的电子结构和动力学
具有明确金属中心的单原子催化剂为探索化学反应的催化活性位点和反应机制提供了独特的机会。然而,由于将对此类位点敏感的操作技术与单原子系统模型相结合的挑战,对反应条件下单原子催化中心的电子和结构动力学的理解仍然有限。在此,在最先进的操作技术的支持下,我们对仅包含铁作为高浓度催化剂的模型催化剂的电化学 CO 2还原反应 (CO 2 RR)过程中的动态结构和电子演化进行了深入研究。-自旋 (HS) Fe(III)N 4中心处于静止状态。Operando 57 Fe 穆斯堡尔和 X 射线吸收光谱清楚地证明了从 HS Fe(III)N 4到 HS Fe(II)N 4中心的变化,并且电解质的电势、CO 2 - 或 Ar 饱和度降低,导致HS Fe(II)N 4中心的不同吸附物和稳定性。通过操作拉曼光谱和循环伏安法,我们发现与铁阳离子中心配位的酞菁(Pc)配体经历了从 Fe(II)Pc 到 Fe(II)Pc – 的氧化还原过程。总之,HS Fe(II)Pc –物质被确定为 CO 2 RR 的催化中间体。此外,理论计算表明,Pc配体的电还原改变了原位生成的HS Fe(II)Pc -物种的d带中心,导致与CO 2的最佳结合强度,从而提高了CO 2的催化性能RR。这项工作为单铁原子材料中反应位点的电子结构和动力学提供了实验和理论证据,并将指导新型高效CO 2 RR催化剂的设计。































 京公网安备 11010802027423号
京公网安备 11010802027423号