Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2023-07-08 , DOI: 10.1016/j.bmcl.2023.129408 Hyo Jae Jung 1 , Duk-Yeon Cho 2 , Jun-Hyuk Han 2 , Ki Dong Park 1 , Dong-Kug Choi 2 , Eunha Kim 1 , Sung-Hwa Yoon 1 , Ju-Young Park 3
|
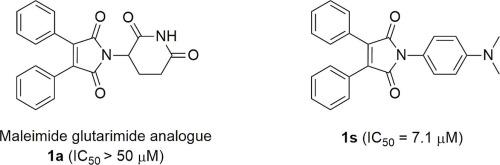
A series of thalidomide analogues, where the fused benzene ring in the phthalimide moiety was converted into two separated diphenyl rings in maleimide moiety and N-aminoglutarimide moiety was replaced by substituted phenyl moiety, were synthesized and evaluated for their NO inhibitory activities on BV2 cells stimulated with lipopolysaccharide (LPS). Among the synthesized compounds, the dimethylaminophenyl analogue 1s (IC50 = 7.1 μM) showed significantly higher inhibitory activity than the glutarimide analogue 1a (IC50 > 50 μM) and suppressed NO production dose-dependently without cytotoxicity. In addition, 1s inhibited the production of pro-inflammatory cytokines and the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) by blocking nuclear factor-kappa B (NF-κB) and p38 MAPK pathways. These results demonstrated that 1s showed good anti-inflammatory activity and could become a leading compound for the treatment of neuroinflammatory diseases.
中文翻译:

1-(4-(二甲氨基)苯基)-3,4-二苯基-1H-吡咯-2,5-二酮类似物的合成及其在脂多糖诱导的BV2细胞中的抗炎活性
合成了一系列沙利度胺类似物,其中邻苯二甲酰亚胺部分中的稠合苯环转化为马来酰亚胺部分中的两个分离的联苯环,并且N-氨基戊二酰亚胺部分被取代的苯基部分取代,并评估了它们对刺激的BV2细胞的NO抑制活性与脂多糖(LPS)。在合成的化合物中,二甲氨基苯基类似物1s (IC 50 = 7.1 μM) 的抑制活性明显高于戊二酰亚胺类似物1a (IC 50 > 50 μM),并且能剂量依赖性地抑制NO产生,且无细胞毒性。此外,1s通过阻断核因子 kappa B (NF-κB) 和 p38 MAPK 通路,抑制促炎细胞因子的产生以及诱导型一氧化氮合酶 (iNOS) 和环氧合酶-2 (COX-2) 的表达。这些结果表明1s表现出良好的抗炎活性,可能成为治疗神经炎症疾病的领先化合物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号