当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-Amino-2-deoxy-1,3-dithioidoglycosides via Organocatalytic Relay Glycosylation of 3-O-Acetyl-2-nitrogalactals
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-07-06 , DOI: 10.1002/cjoc.202300307 Yongyong Wan 1 , Lei Deng 1 , Liming Wang 1 , Yuanhong Tu 1 , Hui Liu 1 , Jian‐song Sun 1, 2 , Qingju Zhang 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-07-06 , DOI: 10.1002/cjoc.202300307 Yongyong Wan 1 , Lei Deng 1 , Liming Wang 1 , Yuanhong Tu 1 , Hui Liu 1 , Jian‐song Sun 1, 2 , Qingju Zhang 1
Affiliation
|
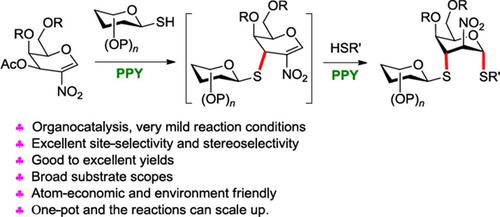
Idose-type glycosides have numerous biological activities and have been widely used as anticoagulant drugs and anti-infection drugs. Thioglycosides have enhanced stability for acid-mediated or enzymatic hydrolysis, and have a wide range of applications in glycobiology and drug development. Herein, we describe an efficient method for site-selective and stereoselective synthesis of potential bioactive 2-amino-2-deoxy-1,3-dithioidoglycosides via organocatalysis sequential C3-Ferrier rearrangement and Michael addition of 3-O-acetyl-2-nitrogalactals. Both stepwise and one-pot protocols were carried out and work well. This unique thio-glycosylation protocol highlighted the various advantages, including (i) mild reaction conditions; (ii) excellent site-selectivity and stereoselectivity, good to excellent yields; (iii) broad substrate scopes; (iv) being atom-economic and environmentally friendly; (v) the reactions can be scaled up.
中文翻译:

通过 3-O-乙酰基-2-硝基半乳糖有机催化中继糖基化合成 2-氨基-2-脱氧-1,3-二硫代糖苷
艾糖型苷类具有多种生物活性,已广泛用作抗凝血药物和抗感染药物。硫代糖苷增强了酸介导或酶水解的稳定性,在糖生物学和药物开发中具有广泛的应用。在此,我们描述了一种通过有机催化连续 C3-Ferrier 重排和 3- O迈克尔加成,位点选择性和立体选择性合成潜在生物活性 2-氨基-2-脱氧-1,3-二硫代糖苷的有效方法。-乙酰基-2-硝基半乳糖。分步方案和一锅法方案均已实施且效果良好。这种独特的硫代糖基化方案突出了各种优点,包括(i)反应条件温和;(ii) 优异的位点选择性和立体选择性,产率良好至优异;(iii) 底物范围广泛;(iv) 原子经济且环境友好;(v) 反应可以扩大规模。
更新日期:2023-07-06

中文翻译:

通过 3-O-乙酰基-2-硝基半乳糖有机催化中继糖基化合成 2-氨基-2-脱氧-1,3-二硫代糖苷
艾糖型苷类具有多种生物活性,已广泛用作抗凝血药物和抗感染药物。硫代糖苷增强了酸介导或酶水解的稳定性,在糖生物学和药物开发中具有广泛的应用。在此,我们描述了一种通过有机催化连续 C3-Ferrier 重排和 3- O迈克尔加成,位点选择性和立体选择性合成潜在生物活性 2-氨基-2-脱氧-1,3-二硫代糖苷的有效方法。-乙酰基-2-硝基半乳糖。分步方案和一锅法方案均已实施且效果良好。这种独特的硫代糖基化方案突出了各种优点,包括(i)反应条件温和;(ii) 优异的位点选择性和立体选择性,产率良好至优异;(iii) 底物范围广泛;(iv) 原子经济且环境友好;(v) 反应可以扩大规模。












































 京公网安备 11010802027423号
京公网安备 11010802027423号