当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single Cu–N4 sites enable atomic Fe clusters with high-performance oxygen reduction reactions
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2023-07-06 , DOI: 10.1039/d3ee00840a
Shuwen Wu 1 , Shang Jiang 1 , Shao-Qing Liu 1 , Xuehai Tan 1 , Ning Chen 2 , Jing-Li Luo 1 , Samir H. Mushrif 1 , Ken Cadien 1 , Zhi Li 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2023-07-06 , DOI: 10.1039/d3ee00840a
Shuwen Wu 1 , Shang Jiang 1 , Shao-Qing Liu 1 , Xuehai Tan 1 , Ning Chen 2 , Jing-Li Luo 1 , Samir H. Mushrif 1 , Ken Cadien 1 , Zhi Li 1
Affiliation
|
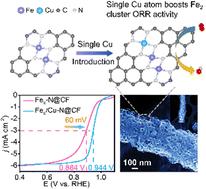
Atomically dispersed Fe–N4 catalysts have proven to be promising alternatives to commercial Pt/C for the oxygen reduction reaction. Most reported Fe–N4 catalysts suffer from inferior O–O bond-breaking capabilities due to superoxo-like O2 adsorption, though the isolated dual-atomic metal site strategy is extensively adopted. Atomic Fe clusters show greater promise for promoting O–O bond cleavage by undergoing peroxo-like O2 adsorption. However, the excessively strong binding strength between Fe clusters and oxygenated intermediates sacrifices the activity. Here, we first report a Fex/Cu–N@CF catalyst with atomic Fe clusters functionalized by adjacent single Cu–N4 sites anchored on a porous carbon nanofiber membrane. Theoretical calculation indicates that the single Cu–N4 sites can modulate the electronic configuration of Fe clusters to reduce the O2* protonation reaction free energy which ultimately enhances the electrocatalytic performance. In particular, the Cu–N4 sites can increase the overlap between the d orbitals of Fe and p orbitals of O to accelerate O–O cleavage in OOH*. As a result, this unique atomic catalyst exhibits a half potential (E1/2) of 0.944 V in alkaline medium, exceeding that of commercial Pt/C, whereas its acidic performance E1/2 = 0.815 V is comparable to that of Pt/C. This work shows the great potential of single atoms for improvements in atomic cluster catalysts.
中文翻译:

单个 Cu-N4 位点使原子 Fe 簇能够进行高性能氧还原反应
原子分散的 Fe-N 4催化剂已被证明是氧还原反应中商业 Pt/C 的有前途的替代品。尽管广泛采用孤立的双原子金属位点策略,但大多数报道的Fe-N 4催化剂由于类超氧O 2吸附而存在较差的O-O键断裂能力。原子 Fe 簇通过经历类过氧 O 2吸附而显示出促进 O-O 键断裂的更大前景。然而,Fe簇和含氧中间体之间的结合强度过强,从而牺牲了活性。在这里,我们首先报道了一种 Fe x /Cu–N@CF 催化剂,其原子 Fe 簇被相邻的单个 Cu–N 4功能化锚定在多孔碳纳米纤维膜上的位点。理论计算表明,单个Cu-N 4位点可以调节Fe团簇的电子构型,降低O 2 * 质子化反应自由能,最终提高电催化性能。特别是,Cu-N 4位点可以增加 Fe 的 d 轨道和 O 的 p 轨道之间的重叠,从而加速 OOH* 中的 O-O 裂解。因此,这种独特的原子催化剂在碱性介质中表现出0.944 V的半电位(E 1/2),超过商业Pt/C,而其酸性性能E 1/2= 0.815 V 与 Pt/C 相当。这项工作显示了单原子在改进原子团簇催化剂方面的巨大潜力。
更新日期:2023-07-06
中文翻译:

单个 Cu-N4 位点使原子 Fe 簇能够进行高性能氧还原反应
原子分散的 Fe-N 4催化剂已被证明是氧还原反应中商业 Pt/C 的有前途的替代品。尽管广泛采用孤立的双原子金属位点策略,但大多数报道的Fe-N 4催化剂由于类超氧O 2吸附而存在较差的O-O键断裂能力。原子 Fe 簇通过经历类过氧 O 2吸附而显示出促进 O-O 键断裂的更大前景。然而,Fe簇和含氧中间体之间的结合强度过强,从而牺牲了活性。在这里,我们首先报道了一种 Fe x /Cu–N@CF 催化剂,其原子 Fe 簇被相邻的单个 Cu–N 4功能化锚定在多孔碳纳米纤维膜上的位点。理论计算表明,单个Cu-N 4位点可以调节Fe团簇的电子构型,降低O 2 * 质子化反应自由能,最终提高电催化性能。特别是,Cu-N 4位点可以增加 Fe 的 d 轨道和 O 的 p 轨道之间的重叠,从而加速 OOH* 中的 O-O 裂解。因此,这种独特的原子催化剂在碱性介质中表现出0.944 V的半电位(E 1/2),超过商业Pt/C,而其酸性性能E 1/2= 0.815 V 与 Pt/C 相当。这项工作显示了单原子在改进原子团簇催化剂方面的巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号