Chinese Journal of Chemical Engineering Pub Date : 2023-07-05 , DOI: 10.1016/j.cjche.2023.06.013 Xiangyang Cui , Xin Zhang , Baoju Wang , Yuqi Sun , Haikui Zou , Guangwen Chu , Yong Luo , Jianfeng Chen
|
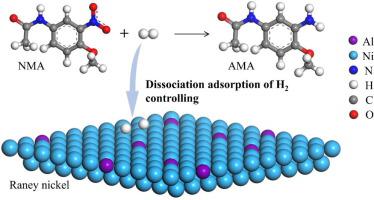
The catalytic hydrogenation of 2-nitro-4-acetylamino anisole (NMA) is a less-polluting and efficient method to produce 2-amino-4-acetamino anisole (AMA). However, the kinetics of catalytic hydrogenation of NMA to AMA remains obscure. In this work, the kinetic models including power-law model and Langmuir-Hinshelwood-Hougen-Watson (LHHW) model of NMA hydrogenation to AMA catalyzed by Raney nickel catalyst were investigated. All experiments were carried out under the elimination of mass transfer resistance within the temperature range of 70–100 °C and the hydrogen pressure of 0.8–1.5 MPa. The reaction was found to follow 0.52-order kinetics with respect to the NMA concentration and 1.10-order kinetics in terms of hydrogen pressure. Based on the LHHW model, the dual-site dissociation adsorption of hydrogen was analyzed to be the rate determining step. The research of intrinsic kinetics of NMA to AMA provides the guidance for the reactor design and inspires the catalyst modification.
中文翻译:

雷尼镍催化剂上 2-硝基-4-乙酰氨基苯甲醚催化加氢生成 2-氨基-4-乙酰氨基苯甲醚的本征动力学
2-硝基-4-乙酰氨基苯甲醚(NMA)催化加氢是一种污染少、高效的生产2-氨基-4-乙酰氨基苯甲醚(AMA)的方法。然而,NMA 催化氢化成 AMA 的动力学仍然不清楚。本工作对雷尼镍催化剂催化NMA加氢生成AMA的动力学模型进行了研究,包括幂律模型和LHHW模型。所有实验均在消除传质阻力的条件下进行,温度范围为70~100℃,氢气压力为0.8~1.5MPa。发现该反应在 NMA 浓度方面遵循 0.52 级动力学,在氢气压力方面遵循 1.10 级动力学。基于LHHW模型,分析氢的双位点解离吸附是速率决定步骤。NMA转化为AMA的本征动力学研究为反应器设计提供了指导,并为催化剂的改性提供了启发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号