当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Emerging investigator series: low doses of electron beam irradiation effectively degrade 1,4-dioxane in water within a few seconds
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2023-07-05 , DOI: 10.1039/d3ew00111c Cheng-Shiuan Lee , Kaushik Londhe , Charles Cooper , Slavica Grdanovska , Arjun Venkatesan
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2023-07-05 , DOI: 10.1039/d3ew00111c Cheng-Shiuan Lee , Kaushik Londhe , Charles Cooper , Slavica Grdanovska , Arjun Venkatesan
|
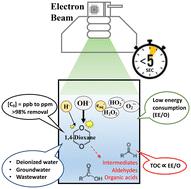
The application of electron beam (e-beam) technology for water treatment has been proposed to be a faster and safer approach to decomposing persistent contaminants in water, because of its ability to rapidly generate high amounts of both oxidizing and reducing reactive species without the addition of chemicals. In this study, we utilized a lab-scale 9 MeV e-beam accelerator to investigate the feasibility of treating 1,4-dioxane in various water matrices in batches with low sample volumes (<90 mL). Very low doses (<5 kGy) and treatment times (<5 s) were sufficient to degrade >98% of 1,4-dioxane within the range of environmentally relevant concentrations (0.1–10 mg L−1), without the need for any sample modification or pH adjustment. Low dissolved oxygen in the solution enhanced the degradation efficiency by 21–23% when treating 1000 mg L−1 of 1,4-dioxane, presumably because of the increased H˙ and O˙− that can react with 1,4-dioxane. Although the degrading intermediates were not fully mineralized to carbon dioxide at the tested doses, the detected intermediates, such as aldehydes and organic acids, were not as persistent as 1,4-dioxane and were more vulnerable to conventional treatment methods and natural attenuation. The slowest reaction rate constant was observed when treating wastewater samples (k = 0.13–0.62 kGy−1), followed by contaminated groundwater (k = 0.16–1.4 kGy−1), suggesting other organics and ions could scavenge the generated reactive species. The electrical energy per order (EE/O) ranged from 0.39 (DI water) to 6.3 (wastewater) kWh m−3 per order, depending on the initial concentration of 1,4-dioxane and water matrix. The EE/O values were comparable to other traditional advanced oxidation processes (AOPs) to treat 1,4-dioxane, suggesting the feasibility of utilizing e-beam to treat contaminated waters. The organic carbon content of the sample positively correlated (R2 >0.9) with the EE/O values and thus can be utilized to predict e-beam treatment performance. Our results show that e-beam radiolysis is a promising technology to treat 1,4-dioxane and could potentially outperform other AOPs and 1,4-dioxane disposal methods (e.g., incineration) in terms of energy consumption and treatment time, leaving no trace of oxidants.
中文翻译:

新兴研究者系列:低剂量电子束照射可在几秒内有效降解水中的1,4-二恶烷
电子束(e-beam)技术在水处理中的应用被认为是分解水中持久性污染物的一种更快、更安全的方法,因为它能够快速产生大量的氧化性和还原性活性物质,而无需添加化学品。在本研究中,我们利用实验室规模的 9 MeV 电子束加速器来研究以低样品量(<90 mL)批量处理各种水基质中的 1,4-二恶烷的可行性。极低的剂量(<5 kGy)和处理时间(<5 s)足以在环境相关浓度范围内(0.1–10 mg L -1)降解>98%的1,4-二恶烷。),无需任何样品修改或 pH 值调整。当处理 1,4-二恶烷 1000 mg L -1时,溶液中的低溶解氧使降解效率提高了 21-23% ,这可能是因为可以与 1,4-二恶烷反应的H˙ 和 O˙ -增加。尽管在测试剂量下降解中间体并未完全矿化为二氧化碳,但检测到的中间体,如醛和有机酸,不像1,4-二恶烷那样持久,并且更容易受到常规处理方法和自然衰减的影响。在处理废水样品时观察到最慢的反应速率常数 ( k = 0.13–0.62 kGy −1 ),其次是受污染的地下水 ( k= 0.16–1.4 kGy −1 ),表明其他有机物和离子可以清除产生的活性物质。每个订单的电能(EE/O)范围为每个订单0.39(去离子水)至6.3(废水)kWh m -3 ,具体取决于1,4-二恶烷和水基质的初始浓度。EE/O 值与处理 1,4-二恶烷的其他传统高级氧化工艺 (AOP) 相当,表明利用电子束处理污染水的可行性。样品的有机碳含量呈正相关(R 2>0.9) 与 EE/O 值,因此可用于预测电子束处理性能。我们的结果表明,电子束辐射分解是一种很有前途的处理 1,4-二恶烷的技术,并且在能源消耗和处理时间方面可能优于其他 AOP 和 1,4-二恶烷处理方法(例如焚烧),且不留痕迹氧化剂。
更新日期:2023-07-05
中文翻译:

新兴研究者系列:低剂量电子束照射可在几秒内有效降解水中的1,4-二恶烷
电子束(e-beam)技术在水处理中的应用被认为是分解水中持久性污染物的一种更快、更安全的方法,因为它能够快速产生大量的氧化性和还原性活性物质,而无需添加化学品。在本研究中,我们利用实验室规模的 9 MeV 电子束加速器来研究以低样品量(<90 mL)批量处理各种水基质中的 1,4-二恶烷的可行性。极低的剂量(<5 kGy)和处理时间(<5 s)足以在环境相关浓度范围内(0.1–10 mg L -1)降解>98%的1,4-二恶烷。),无需任何样品修改或 pH 值调整。当处理 1,4-二恶烷 1000 mg L -1时,溶液中的低溶解氧使降解效率提高了 21-23% ,这可能是因为可以与 1,4-二恶烷反应的H˙ 和 O˙ -增加。尽管在测试剂量下降解中间体并未完全矿化为二氧化碳,但检测到的中间体,如醛和有机酸,不像1,4-二恶烷那样持久,并且更容易受到常规处理方法和自然衰减的影响。在处理废水样品时观察到最慢的反应速率常数 ( k = 0.13–0.62 kGy −1 ),其次是受污染的地下水 ( k= 0.16–1.4 kGy −1 ),表明其他有机物和离子可以清除产生的活性物质。每个订单的电能(EE/O)范围为每个订单0.39(去离子水)至6.3(废水)kWh m -3 ,具体取决于1,4-二恶烷和水基质的初始浓度。EE/O 值与处理 1,4-二恶烷的其他传统高级氧化工艺 (AOP) 相当,表明利用电子束处理污染水的可行性。样品的有机碳含量呈正相关(R 2>0.9) 与 EE/O 值,因此可用于预测电子束处理性能。我们的结果表明,电子束辐射分解是一种很有前途的处理 1,4-二恶烷的技术,并且在能源消耗和处理时间方面可能优于其他 AOP 和 1,4-二恶烷处理方法(例如焚烧),且不留痕迹氧化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号