Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2023-07-04 , DOI: 10.1016/j.psep.2023.06.091 Albandary Almahri , Moataz Morad , Meshari M. Aljohani , Nada M. Alatawi , Fawaz A. Saad , Hana M. Abumelha , Mohamed G. El-Desouky , Ashraf A. El-Bindary
|
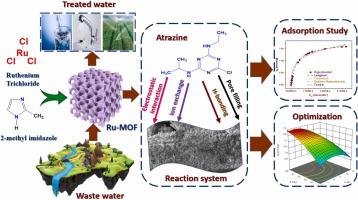
The current research describes the synthesis, characterization, and application of a newly developed metal organic framework grounded on ruthenium (Ru-MOF) for the elimination of hazardous chemicals atrazine (AZ) from water. The zeta potential, N2 adsorption/desorption, SEM, TEM, XPS, and FTIR Spectroscopy were used to analyze the Ru-MOF. Ru-MOF has a high surface area of 1058.62 m2.g−1 and micropores with a pore volume of 0.74 cm3.g−1 were produced by the characterization analyses. Additionally, it was discovered that atrazine adsorption produced good results at pH 4 and an adsorbent mass of 0.8 g per liter of solution. The greatest successful fit with the equilibrium facts was provided by the Langmuir model isothermally and Pseudo-Second-Order kinetically at 298 K, with a maximum adsorption capacity of 382.7 mg.g−1. A spontaneous endothermic process was validated using the thermodynamic characteristics. The results of kinetic investigations, which were fitted to a pseudo-second-order model, displayed that equilibrium was got after 90 min. In terms of adsorption kinetics, the experimental results can be represented by the linear driving force model. Additionally, the projected adsorption data of the model agree with the results of the experiment. Box Behnken design study may also show the ideal circumstances for greater atrazine elimination by Ru-MOF. Hydrophobic, π–π interactions and pore filling processes that take place at the surfaces may be used to govern the adsorption mechanisms. When the adsorption capacity was compared to the literature review, it was determined that the Ru-MOF had the best adsorption capacity out of all the preceding adsorbents. Check the adsorbent's regeneration as it demonstrated good efficiency for five cycles, which was deemed a significant cost-saving benefit.
中文翻译:

使用钌基金属有机骨架从水环境中回收莠去津
目前的研究描述了新开发的基于钌的金属有机框架(Ru-MOF)的合成、表征和应用,用于消除水中的危险化学品莠去津(AZ)。使用zeta电位、N 2吸附/解吸、SEM、TEM 、XPS和FTIR光谱来分析Ru-MOF。Ru-MOF具有1058.62 m 2 .g -1的高表面积和孔体积为0.74 cm 3 .g -1的微孔是通过表征分析产生的。此外,还发现莠去津吸附在pH 4 和吸附剂质量为每升溶液0.8 克时产生良好的结果。Langmuir 模型在 298 K 的等温和伪二级动力学上最成功地拟合了平衡事实,最大吸附容量为 382.7 mg.g -1 。使用热力学特性验证了自发吸热过程。拟合准二阶模型的动力学研究结果表明,90 分钟后达到平衡。就吸附动力学而言,实验结果可以表示为线性驱动力模型。此外,模型的预测吸附数据与实验结果一致。Box Behnken 设计研究也可能表明 Ru-MOF 更大程度地消除莠去津的理想环境。表面发生的疏水性、π-π相互作用和孔隙填充过程可用于控制吸附机制。当吸附容量与文献综述进行比较时,确定Ru-MOF在所有先前的吸附剂中具有最好的吸附容量。检查吸附剂的再生,因为它在五个循环中表现出良好的效率,这被认为是一个显着的成本节约效益。































 京公网安备 11010802027423号
京公网安备 11010802027423号