European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-06-30 , DOI: 10.1016/j.ejmech.2023.115616 Yongjin Hao 1 , Jiawan Ma 1 , Jin Wang 1 , Xiaoliang Yu 2 , Zhanhui Li 1 , Shuwei Wu 1 , Sheng Tian 1 , Haikuo Ma 1 , Sudan He 3 , Xiaohu Zhang 1
|
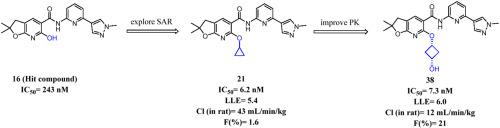
Interleukin-1 receptor-associated kinase 4 (IRAK4) is a key regulator to control downstream NF-κB and MAPK signals in the innate immune response and has been proposed as a therapeutic target for the treatment of inflammatory and autoimmune diseases. Herein, a series of IRAK4 inhibitors based on a dihydrofuro[2,3-b]pyridine scaffold was developed. Structural modifications of the screening hit 16 (IC50 = 243 nM) led to IRAK4 inhibitors with improved potency but high clearance (Cl) and poor oral bioavailability, as exemplified by compound 21 (IC50 = 6.2 nM, Cl = 43 ml/min/kg, F = 1.6%, LLE = 5.4). Structure modification aimed at improving LLE and reducing clearance identified compound 38. Compound 38 showed significantly improved clearance while maintained excellent biochemical potency against IRAK4 (IC50 = 7.3 nM, Cl = 12 ml/min/kg, F = 21%, LLE = 6.0). Importantly, compound 38 had favorable in vitro safety and ADME profiles. Furthermore, compound 38 reduced the in vitro production of pro-inflammatory cytokines in both mouse iBMDMs and human PBMCs and was orally efficacious in the inhibition of serum TNF-α secretion in LPS-induced mouse model. These findings suggested that compound 38 has development potential as an IRAK4 inhibitor for the treatment of inflammatory and autoimmune disorders.
中文翻译:

二氢呋喃并[2,3-b]吡啶衍生物的合成和作为有效IRAK4抑制剂的评价
Interleukin-1 受体相关激酶 4 (IRAK4) 是先天免疫反应中控制下游 NF-κB 和 MAPK 信号的关键调节因子,已被提议作为治疗炎症和自身免疫性疾病的治疗靶点。在此,开发了一系列基于二氢呋喃并[2,3- b ]吡啶支架的IRAK4抑制剂。筛选命中16 (IC 50 = 243 nM) 的结构修饰导致 IRAK4 抑制剂的效力提高,但清除率 (Cl) 高且口服生物利用度差,如化合物21 (IC 50 = 6.2 nM,Cl = 43 ml/min) /kg,F = 1.6%,LLE = 5.4)。结构修饰旨在改善 LLE 并减少已确定的化合物38 的间隙。化合物38显示出显着改善的清除率,同时保持优异的针对IRAK4的生化效力(IC 50 = 7.3 nM,Cl = 12 ml/min/kg,F = 21%,LLE = 6.0)。重要的是,化合物38具有良好的体外安全性和 ADME 特征。此外,化合物38可以减少小鼠 iBMDM 和人 PBMC 中促炎细胞因子的体外产生,并且口服可有效抑制 LPS 诱导的小鼠模型中血清 TNF-α 的分泌。这些发现表明化合物38具有作为 IRAK4 抑制剂治疗炎症和自身免疫性疾病的开发潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号