当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of C—N Axial Chirality N-Arylindoles via Pd(II)-Catalyzed Free Amine-Directed Atroposelective C—H Olefination†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-07-03 , DOI: 10.1002/cjoc.202300355
Lei Wang 1 , Wen‐Kui Yuan 1 , Zhen‐Kai Wang 1 , Jun Luo 1 , Tao Zhou 1 , Bing‐Feng Shi 1, 2, 3
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-07-03 , DOI: 10.1002/cjoc.202300355
Lei Wang 1 , Wen‐Kui Yuan 1 , Zhen‐Kai Wang 1 , Jun Luo 1 , Tao Zhou 1 , Bing‐Feng Shi 1, 2, 3
Affiliation
|
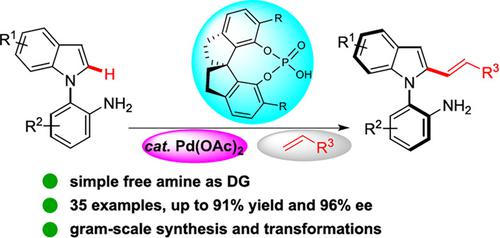
Axially chiral N-arylindoles bearing a stereogenic C—N axis are unique important scaffolds in natural products, advance materials, pharmaceuticals and privileged chiral ligands or catalysts. Herein, we report the direct synthesis of C—N axially chiral N-arylindoles through a Pd-catalyzed free amine-directed atroposelective C—H olefination enabled by a spiro phosphoric acid (SPA) ligand. A wide range of enantioenriched N-aromatic amine indoles were obtained in high yields with good enantioselectivities (35 examples, up to 91% yield and up to 96% ee). The chiral products with free amine group offer an effective functional handle for down-stream diversity-oriented synthesis.

中文翻译:

通过 Pd(II) 催化的游离胺定向 Atroposelective C-H 烯化合成 C-N 轴向手性 N-芳基吲哚†
带有立构CN轴的轴向手性N-芳基吲哚是天然产物、先进材料、药物和特殊手性配体或催化剂中独特的重要支架。在此,我们报道了通过螺磷酸(SPA)配体实现的Pd催化的游离胺定向的C-H烯化直接合成CN轴向手性N-芳基吲哚。以高产率和良好的对映选择性获得了多种对映体富集的N-芳香胺吲哚(35 个实例,产率高达 91%,ee 高达 96%)。具有游离胺基的手性产物为下游多样性导向的合成提供了有效的功能手柄。
更新日期:2023-07-03

中文翻译:

通过 Pd(II) 催化的游离胺定向 Atroposelective C-H 烯化合成 C-N 轴向手性 N-芳基吲哚†
带有立构CN轴的轴向手性N-芳基吲哚是天然产物、先进材料、药物和特殊手性配体或催化剂中独特的重要支架。在此,我们报道了通过螺磷酸(SPA)配体实现的Pd催化的游离胺定向的C-H烯化直接合成CN轴向手性N-芳基吲哚。以高产率和良好的对映选择性获得了多种对映体富集的N-芳香胺吲哚(35 个实例,产率高达 91%,ee 高达 96%)。具有游离胺基的手性产物为下游多样性导向的合成提供了有效的功能手柄。


































 京公网安备 11010802027423号
京公网安备 11010802027423号