当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetraphenylporphyrin electrocatalysts for the hydrogen evolution reaction: applicability of molecular volcano plots to experimental operating conditions
Dalton Transactions ( IF 3.5 ) Pub Date : 2023-07-04 , DOI: 10.1039/d3dt01250f Felicia Zaar 1 , C Moyses Araujo 2, 3 , Rikard Emanuelsson 4 , Maria Strømme 1 , Martin Sjödin 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2023-07-04 , DOI: 10.1039/d3dt01250f Felicia Zaar 1 , C Moyses Araujo 2, 3 , Rikard Emanuelsson 4 , Maria Strømme 1 , Martin Sjödin 1
Affiliation
|
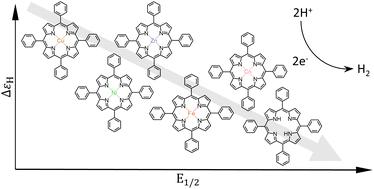
Recent years have seen an increasing interest in molecular electrocatalysts for the hydrogen evolution reaction (HER). Efficient hydrogen evolution would play an important role in a sustainable fuel economy, and molecular systems could serve as highly specific and tunable alternatives to traditional noble metal surface catalysts. However, molecular catalysts are currently mostly used in homogeneous setups, where quantitative evaluation of catalytic activity is non-standardized and cumbersome, in particular for multistep, multielectron processes. The molecular design community would therefore be well served by a straightforward model for prediction and comparison of the efficiency of molecular catalysts. Recent developments in this area include attempts at applying the Sabatier principle and the volcano plot concept – popular tools for comparing metal surface catalysts – to molecular catalysis. In this work, we evaluate the predictive power of these tools in the context of experimental operating conditions, by applying them to a series of tetraphenylporphyrins employed as molecular electrocatalysts of the HER. We show that the binding energy of H and the redox chemistry of the porphyrins depend solely on the electron withdrawing ability of the central metal ion, and that the thermodynamics of the catalytic cycle follow a simple linear free energy relation. We also find that the catalytic efficiency of the porphyrins is almost exclusively determined by reaction kinetics and therefore cannot be explained by thermodynamics alone. We conclude that the Sabatier principle, linear free energy relations and molecular volcano plots are insufficient tools for predicting and comparing activity of molecular catalysts, and that experimentally useful information of catalytic performance can still only be obtained through detailed knowledge of the catalytic pathway for each individual system.
中文翻译:

用于析氢反应的四苯基卟啉电催化剂:分子火山图对实验操作条件的适用性
近年来,人们对用于析氢反应(HER)的分子电催化剂越来越感兴趣。高效的析氢将在可持续的燃料经济中发挥重要作用,分子系统可以作为传统贵金属表面催化剂的高度特异性和可调的替代品。然而,分子催化剂目前主要用于均相装置,其中催化活性的定量评估是非标准化且繁琐的,特别是对于多步骤、多电子过程。因此,分子设计界可以通过一个简单的模型来预测和比较分子催化剂的效率。该领域的最新进展包括尝试将萨巴蒂尔原理和火山图概念(用于比较金属表面催化剂的流行工具)应用于分子催化。在这项工作中,我们通过将这些工具应用于一系列用作 HER 分子电催化剂的四苯基卟啉,评估了这些工具在实验操作条件下的预测能力。我们表明,H 的结合能和卟啉的氧化还原化学仅取决于中心金属离子的吸电子能力,并且催化循环的热力学遵循简单的线性自由能关系。我们还发现卟啉的催化效率几乎完全由反应动力学决定,因此不能仅用热力学来解释。我们的结论是,萨巴蒂尔原理、线性自由能关系和分子火山图不足以作为预测和比较分子催化剂活性的工具,并且仍然只能通过对每个个体的催化途径的详细了解来获得催化性能的实验有用信息系统。
更新日期:2023-07-04
中文翻译:

用于析氢反应的四苯基卟啉电催化剂:分子火山图对实验操作条件的适用性
近年来,人们对用于析氢反应(HER)的分子电催化剂越来越感兴趣。高效的析氢将在可持续的燃料经济中发挥重要作用,分子系统可以作为传统贵金属表面催化剂的高度特异性和可调的替代品。然而,分子催化剂目前主要用于均相装置,其中催化活性的定量评估是非标准化且繁琐的,特别是对于多步骤、多电子过程。因此,分子设计界可以通过一个简单的模型来预测和比较分子催化剂的效率。该领域的最新进展包括尝试将萨巴蒂尔原理和火山图概念(用于比较金属表面催化剂的流行工具)应用于分子催化。在这项工作中,我们通过将这些工具应用于一系列用作 HER 分子电催化剂的四苯基卟啉,评估了这些工具在实验操作条件下的预测能力。我们表明,H 的结合能和卟啉的氧化还原化学仅取决于中心金属离子的吸电子能力,并且催化循环的热力学遵循简单的线性自由能关系。我们还发现卟啉的催化效率几乎完全由反应动力学决定,因此不能仅用热力学来解释。我们的结论是,萨巴蒂尔原理、线性自由能关系和分子火山图不足以作为预测和比较分子催化剂活性的工具,并且仍然只能通过对每个个体的催化途径的详细了解来获得催化性能的实验有用信息系统。

































 京公网安备 11010802027423号
京公网安备 11010802027423号