Nano Research ( IF 9.5 ) Pub Date : 2023-07-01 , DOI: 10.1007/s12274-023-5752-5 Junhua Li , Yi Jiang , Xu Zhang , Yidan Huo , Fanglin Du , Yongxiao Tuo , Zhiyan Guo , Dawei Chen , Shenghua Chen
|
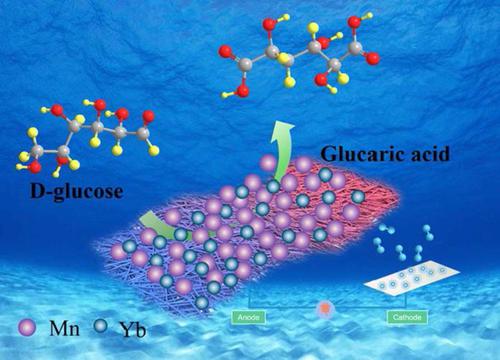
The electrooxidation of the alcohol and aldehyde molecules instead of water coupled with H2 production has been proven to be effective for producing high-value fine chemicals under alkaline conditions. It is also noteworthy that under acidic conditions, the stability of non-noble metal water oxidation catalysts remains a great challenge due to the lattice oxygen mechanism. Hence, we coupled the biomass-derived glucose oxidation for high-value D-glucaric acid (GRA) with ultra-durable hydrogen in acid solution over a Yb-MnO2 catalyst. The Mn3+ regulated by Yb atoms doped in MnO2 can effectively optimize the adsorption and desorption processes of the alcohol and aldehyde group and improve the intrinsic activity but cannot for H2O. The catalyst exhibited extremely high activity and stability after 50 h for glucose oxidation, inhibiting the lattice oxygen process and MnO4− formation, while the activity was quickly lost within 0.5 h for water oxidation. Density functional theory (DFT) calculations further demonstrated that glucose oxidation reaction proceeds preferentially due to the oxidation of aldehyde group with lower adsorption-free energy (−0.4 eV) than water (ΔG > 0 eV), avoiding the lattice oxygen mechanism. This work suggests that biomass-derived glucose oxidation not only provides a cost-effective approach for high-value chemicals, but also shows an extremely potential as an alternative to acidic oxygen evolution reaction (OER) for ultradurable H2 production.
中文翻译:

在酸性溶液中通过 Mn(III) 高效耦合葡萄糖氧化生成高价值 D-葡萄糖酸与超耐久氢
醇和醛分子代替水的电氧化加上H 2 的生产已被证明对于在碱性条件下生产高价值精细化学品是有效的。还值得注意的是,在酸性条件下,由于晶格氧机制,非贵金属水氧化催化剂的稳定性仍然是一个巨大的挑战。因此,我们在 Yb-MnO 2催化剂上将生物质衍生的葡萄糖氧化产生高价值 D-葡萄糖酸 (GRA) 与酸性溶液中的超耐用氢结合起来。MnO 2中掺杂Yb原子调控的Mn 3+可以有效优化醇基和醛基的吸附和解吸过程,提高内在活性,但不能有效地抑制H2 O。催化剂在葡萄糖氧化50小时后表现出极高的活性和稳定性,抑制晶格氧过程和MnO 4−形成,而在水氧化0.5小时内活性迅速丧失。密度泛函理论(DFT)计算进一步表明,由于醛基的氧化具有比水更低的吸附自由能(-0.4 eV)(Δ G > 0 eV),葡萄糖氧化反应优先进行,避免了晶格氧机制。这项工作表明,生物质衍生的葡萄糖氧化不仅为高价值化学品提供了一种具有成本效益的方法,而且还显示出作为酸性析氧反应(OER)的替代品用于超耐用H 2 生产的巨大潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号