当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-07-03 , DOI: 10.1021/acs.chemmater.3c00801
Terry J. Frankcombe 1 , Yun Liu 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-07-03 , DOI: 10.1021/acs.chemmater.3c00801
Terry J. Frankcombe 1 , Yun Liu 2
Affiliation
|
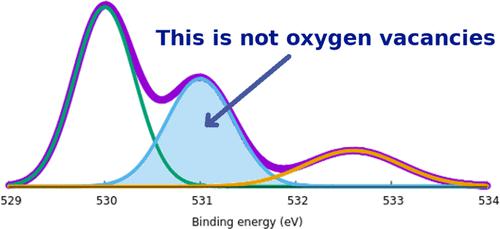
X-ray photoelectron spectroscopy (XPS) is widely used to determine the chemical and electronic states of atoms within a material. However, it is often complex to interpret the O 1s region in metal oxides, where an ∼531 eV binding energy feature appears between lattice oxygen (∼530 eV) and oxygen-containing surface species (∼532 eV). This feature has been vaguely ascribed to oxygen vacancies or oxygen deficient regions for many decades. This work employs full-potential density functional theory to calculate the binding energies of the O 1s electrons under two- and three-dimensional periodic boundary conditions as a probe of expected XPS spectra. ZnO is used as an example system. Both bulk crystal regions containing a range of oxygen defects and slabs with a range of surface terminations and functionalizations have been considered. The slabs considered are mostly {1010} and {1120} surfaces that are not expected to be reconstructed from the cleaved bulk structure. The resulting O 1s binding energies show no signature for oxygen defects in bulk regions. Furthermore, the 531 eV binding energy feature often ascribed to oxygen vacancies or oxygen deficient regions can instead be readily explained by the O 1s electrons from water molecules strongly bound to the exposed ZnO surface (i.e., chemisorbed, as distinct from more loosely bound water) or surface oxygen passivated with hydrogen. This work will rectify many misinterpretations of XPS data of the O 1s region in metal oxides, provide guidance for precisely understanding the oxygen states of a material, and subsequently enable the real origin of material properties to be revealed.
中文翻译:

ZnO 氧 1s X 射线光电子能谱的解释
X 射线光电子能谱 (XPS) 广泛用于确定材料内原子的化学和电子状态。然而,解释金属氧化物中的 O 1s 区域通常很复杂,其中晶格氧 (~530 eV) 和含氧表面物质 (~532 eV) 之间出现~531 eV 结合能特征。几十年来,这一特征一直被模糊地归因于氧空位或缺氧区域。这项工作采用全势密度泛函理论来计算二维和三维周期性边界条件下 O 1s 电子的结合能,作为预期 XPS 光谱的探针。ZnO 用作示例系统。包含一系列氧缺陷的块状晶体区域和具有一系列表面终端和功能化的板都已被考虑。1 0} 和 {11 2 0} 表面预计不会从劈裂的块体结构中重建。所得的 O 1s 结合能没有显示体区域中氧缺陷的特征。此外,通常归因于氧空位或缺氧区域的 531 eV 结合能特征可以很容易地用来自水分子的 O 1s 电子来解释,该水分子与暴露的 ZnO 表面牢固结合(即化学吸附,与更松散结合的水不同)或用氢钝化的表面氧。这项工作将纠正对金属氧化物中O 1s 区域XPS数据的许多误解,为精确理解材料的氧态提供指导,从而揭示材料特性的真正起源。
更新日期:2023-07-03
中文翻译:

ZnO 氧 1s X 射线光电子能谱的解释
X 射线光电子能谱 (XPS) 广泛用于确定材料内原子的化学和电子状态。然而,解释金属氧化物中的 O 1s 区域通常很复杂,其中晶格氧 (~530 eV) 和含氧表面物质 (~532 eV) 之间出现~531 eV 结合能特征。几十年来,这一特征一直被模糊地归因于氧空位或缺氧区域。这项工作采用全势密度泛函理论来计算二维和三维周期性边界条件下 O 1s 电子的结合能,作为预期 XPS 光谱的探针。ZnO 用作示例系统。包含一系列氧缺陷的块状晶体区域和具有一系列表面终端和功能化的板都已被考虑。1 0} 和 {11 2 0} 表面预计不会从劈裂的块体结构中重建。所得的 O 1s 结合能没有显示体区域中氧缺陷的特征。此外,通常归因于氧空位或缺氧区域的 531 eV 结合能特征可以很容易地用来自水分子的 O 1s 电子来解释,该水分子与暴露的 ZnO 表面牢固结合(即化学吸附,与更松散结合的水不同)或用氢钝化的表面氧。这项工作将纠正对金属氧化物中O 1s 区域XPS数据的许多误解,为精确理解材料的氧态提供指导,从而揭示材料特性的真正起源。

































 京公网安备 11010802027423号
京公网安备 11010802027423号