当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ethylene Electrooxidation to 2-Chloroethanol in Acidic Seawater with Natural Chloride Participation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-03 , DOI: 10.1021/jacs.3c05114
Linsen Huang 1 , Pengtang Wang 1 , Yunling Jiang 1 , Kenneth Davey 1 , Yao Zheng 1 , Shi-Zhang Qiao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-03 , DOI: 10.1021/jacs.3c05114
Linsen Huang 1 , Pengtang Wang 1 , Yunling Jiang 1 , Kenneth Davey 1 , Yao Zheng 1 , Shi-Zhang Qiao 1
Affiliation
|
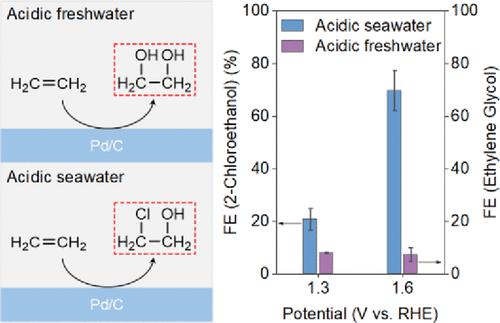
Ethylene oxidation to oxygenates via electrocatalysis is practically promising because of less energy input and CO2 output compared with traditional thermal catalysis. However, current ethylene electrooxidation reaction (EOR) is limited to alkaline and neutral electrolytes to produce acetaldehyde and ethylene glycol, significantly limiting cell energy efficiency. Here, we report for the first time an EOR to 2-chloroethanol product in a strongly acidic environment with natural seawater as an electrolyte. We demonstrate a 2-chloroethanol Faradaic efficiency (FE) of ∼70% with a low electrical energy consumption of ∼1.52 × 10–3 kWh g–1 over a commercial Pd catalyst. We establish a mechanism to evidence that 2-chloroethanol is produced at low potentials via direct interaction of adsorbed chloride anions (*Cl) with ethylene reactant because of the high coverage of *Cl during reaction. Importantly, this differs from the accepted multiple step mechanism of subsequent chlorine oxidation and ethylene chlorination reactions at high potentials. With highly active Cl– participation, the production rate for 2-chloroethanol in acidic seawater is a high 26.3 g m–2 h–1 at 1.6 V operation. Significantly, we show that this is 223 times greater than that for ethylene glycol generation in acidic freshwater. We demonstrate chloride-participated EOR in a proton exchange membrane electrolyzer that exhibits a 68% FE for 2-chloroethanol at 2.2 V operation in acidic seawater. This new understanding can be used for designing selective anode oxidation reactions in seawater under mild conditions.
中文翻译:

天然氯化物参与的酸性海水中乙烯电氧化制2-氯乙醇
通过电催化将乙烯氧化成含氧化合物实际上是有前景的,因为与传统的热催化相比,能量输入和CO 2输出更少。然而,目前的乙烯电氧化反应(EOR)仅限于碱性和中性电解质产生乙醛和乙二醇,严重限制了电池的能源效率。在这里,我们首次报道了在强酸性环境中以天然海水作为电解质的 EOR 至 2-氯乙醇产品。我们证明,在商用 Pd 催化剂上,2-氯乙醇法拉第效率 (FE) 约为 70%,电能消耗低至 ∼1.52 × 10 –3 kWh g –1 。我们建立了一种机制来证明 2-氯乙醇是通过吸附的氯阴离子 (*Cl) 与乙烯反应物的直接相互作用在低电势下产生的,因为反应过程中 *Cl 的覆盖率很高。重要的是,这不同于公认的高电位下随后的氯氧化和乙烯氯化反应的多步骤机制。在高活性 Cl – 的参与下,酸性海水中 2-氯乙醇的产率在 1.6 V 运行时高达 26.3 gm –2 h –1。值得注意的是,我们发现这比酸性淡水中乙二醇的生成量高 223 倍。我们在质子交换膜电解槽中演示了氯化物参与的 EOR,该电解槽在酸性海水中以 2.2 V 运行时,2-氯乙醇的 FE 为 68%。这一新的认识可用于设计温和条件下海水中的选择性阳极氧化反应。
更新日期:2023-07-03
中文翻译:

天然氯化物参与的酸性海水中乙烯电氧化制2-氯乙醇
通过电催化将乙烯氧化成含氧化合物实际上是有前景的,因为与传统的热催化相比,能量输入和CO 2输出更少。然而,目前的乙烯电氧化反应(EOR)仅限于碱性和中性电解质产生乙醛和乙二醇,严重限制了电池的能源效率。在这里,我们首次报道了在强酸性环境中以天然海水作为电解质的 EOR 至 2-氯乙醇产品。我们证明,在商用 Pd 催化剂上,2-氯乙醇法拉第效率 (FE) 约为 70%,电能消耗低至 ∼1.52 × 10 –3 kWh g –1 。我们建立了一种机制来证明 2-氯乙醇是通过吸附的氯阴离子 (*Cl) 与乙烯反应物的直接相互作用在低电势下产生的,因为反应过程中 *Cl 的覆盖率很高。重要的是,这不同于公认的高电位下随后的氯氧化和乙烯氯化反应的多步骤机制。在高活性 Cl – 的参与下,酸性海水中 2-氯乙醇的产率在 1.6 V 运行时高达 26.3 gm –2 h –1。值得注意的是,我们发现这比酸性淡水中乙二醇的生成量高 223 倍。我们在质子交换膜电解槽中演示了氯化物参与的 EOR,该电解槽在酸性海水中以 2.2 V 运行时,2-氯乙醇的 FE 为 68%。这一新的认识可用于设计温和条件下海水中的选择性阳极氧化反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号