Molecular Catalysis ( IF 3.9 ) Pub Date : 2023-06-30 , DOI: 10.1016/j.mcat.2023.113335 Yaning Zhang , Wenhai Xu , Anuj Kumar , Hao Sun , Aiqing Cao , Zhuang Zhang , Zheheng Jiang , Zuoyin Yang , Juncai Dong , Yaping Li
|
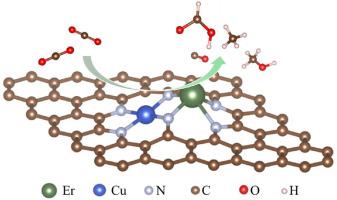
A high atomic utilization and excellent catalytic activity distinguish atomic dispersion catalysts from the others, which are frequently utilized in CO2 reduction. Compared with single-atom catalysts (SACs), diatomic catalysts (DACs) have more dispersed active sites and different electronic structures, which could show different catalytic properties. Here, the CO2 reduction reaction (CO2RR) to C1 products process was systematically investigated for five different graphene-supported catalysts, including monatomic CuNC and ErNC, diatomic CuNC-ErNC, CuErNC-I, and CuErNC-II, using first principles calculations. Their free-energy diagrams were created, and the limit-potential /overpotential of different products were determined. It was shown that the minimum overpotential for CO2RR to CO/CH4 was 0.28/0.91 V on CuErNC-I(Er), to HCOOH was 1.01 V on CuErNC-I(Cu/Er), and to CH3OH was 0.81 V on CuErNC-I(Cu), respectively. At the same time, CuErNC-I displayed better electrochemical activity than others, and CuNC-ErNC had high selectivity comparing CO2RR with hydrogen evolution reaction (HER). This work provided a successful example to design high performance and selectivity catalysts by anchoring metal dimers to N-doped graphene substrates.
中文翻译:

氮掺杂石墨烯支持的 Cu/Er 的 CO2 还原性能:第一原理研究
高原子利用率和优异的催化活性使原子分散催化剂区别于其他常用于CO 2还原的催化剂。与单原子催化剂(SAC)相比,双原子催化剂(DAC)具有更分散的活性位点和不同的电子结构,从而表现出不同的催化性能。这里,CO 2还原反应(CO 2使用第一原理计算,系统地研究了五种不同的石墨烯负载催化剂(包括单原子 CuNC 和 ErNC、双原子 CuNC-ErNC、CuErNC-I 和 CuErNC-II)从 RR)到 C1 产品的过程。创建了它们的自由能图,并确定了不同产品的极限电势/超电势。结果表明,CO 2 RR在CuErNC-I(Er)上对CO/CH 4的最小过电势为0.28/0.91 V,在CuErNC-I(Cu/Er)上对HCOOH的最小过电势为1.01 V,对CH 3 OH的最小过电势为CuErNC-I(Cu) 上分别为 0.81 V。同时,CuErNC-I表现出比其他材料更好的电化学活性,且CuNC-ErNC与CO 2相比具有较高的选择性RR 与析氢反应 (HER)。这项工作为通过将金属二聚体锚定到氮掺杂石墨烯基底上来设计高性能和选择性催化剂提供了成功的例子。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号