Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-06-29 , DOI: 10.1016/j.apcatb.2023.123043 Lei Wang , Wen-Wen Tian , Wenlin Zhang , Fengshou Yu , Zhong-Yong Yuan
|
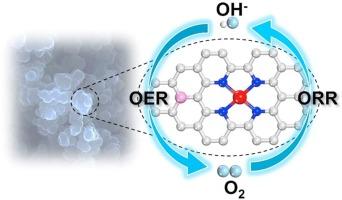
Atomically dispersed catalysts have been widely explored for some reactions, but it remains a challenge to modulate the electronic structure of the center metal for enhancing the electrochemical activity. Herein, the electrochemical activity of copper single-atom catalyst (Cu SAC) was significantly improved by doping secondary heteroatomic phosphorus to engineer the d-band center of Cu atoms, which was named Cu SAC/P. In alkaline media, the Cu SAC/P-700 catalyst exhibited excellent oxygen reduction reaction (ORR) performance with a higher half-wave potential than Cu SAC-700, even better than the state-of-the-art 20 wt% Pt/C 20 mV. The oxygen electroactivity coefficient was only 0.76 V, achieving good performance in zinc-air battery tests. Density functional theory calculations suggested that phosphorus effectively modulated the density of states and the d-band center of Cu atoms. It improved the adsorption of the reaction intermediates and facilitated to trap them on Cu atoms, thus boosting the ORR activity of the catalyst.
中文翻译:

通过二次杂原子磷调制改造 d 带中心来提高铜原子的氧电催化性能
原子分散的催化剂已被广泛探索用于一些反应,但调节中心金属的电子结构以增强电化学活性仍然是一个挑战。在此,通过掺杂二级杂原子磷来设计d,显着提高了铜单原子催化剂(Cu SAC)的电化学活性。Cu原子的-能带中心,命名为Cu SAC/P。在碱性介质中,Cu SAC/P-700催化剂表现出优异的氧还原反应(ORR)性能,其半波电位比Cu SAC-700更高,甚至优于最先进的20 wt% Pt/ C 20毫伏。氧电活系数仅为0.76 V,在锌空气电池测试中取得了良好的性能。密度泛函理论计算表明,磷有效地调节了铜原子的态密度和d带中心。它改善了反应中间体的吸附并有利于将它们捕获在铜原子上,从而提高了催化剂的ORR活性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号