当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-valent cobalt-oxo species triggers singlet oxygen for rapid contaminants degradation along with mild peroxymonosulfate decomposition in single Co atom-doped g-C3N4
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-06-30 , DOI: 10.1016/j.cej.2023.144531
Yixiao Zou , Jie Li , Jie Tan , Lai Lyu , Shangyi Li , Yuhui Wang , Yong Lu , Xiaobiao Zhu , Tingting Zhang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-06-30 , DOI: 10.1016/j.cej.2023.144531
Yixiao Zou , Jie Li , Jie Tan , Lai Lyu , Shangyi Li , Yuhui Wang , Yong Lu , Xiaobiao Zhu , Tingting Zhang
|
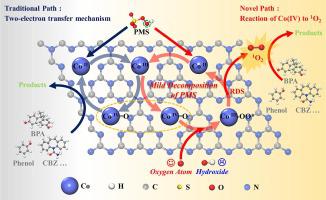
Tailoring the interfacial reaction pathway and low consumption strategy for the high efficiency Fenton-like processes is environmentally desirable but still challenging. Herein, single Co atom-doped g-C3 N4 was prepared for peroxymonosulfate (PMS) activation to reveal the interfacial reaction of high-valent cobalt-oxo species [Co(IV) = O] to singlet oxygen (1 O2 ) and construct an energy efficient Fenton-like system. Experimental results showed that the system could achieve 99.6% carbamazepine removal with k obs of 0.355 min−1 , while the decomposition of PMS was mild (0.0988 and 0.00917 min−1 in 0–2 and 2–30 min, respectively). Mechanistic studies revealed the reaction of Co(IV) = O to 1 O2 , which moderated the reduction of Co sites, thus slowing the decomposition rate of PMS. But the synergistic effect of 1 O2 and Co(IV) = O maintained the high activity of the system. Combined with first-principles calculations, the optimal evolution path of PMS was PMS → OH*→Co(IV) = O → OO*→1 O2 , accompanied by the Co(II)/Co(III)/Co(IV) redox cycle. The work proposed a new interfacial reaction in term of Co(IV) = O triggered 1 O2 generation, resulting in the construction of an energy-efficient Fenton-like system and provided a novel idea for the development of efficient and low consumption water purification technology.
中文翻译:

高价钴-氧代物质触发单线态氧,在单个 Co 原子掺杂 g-C3N4 中触发单线态氧快速降解污染物以及温和的过氧一硫酸盐分解
为高效 Fenton 类过程定制界面反应途径和低消耗策略是环境可取的,但仍然具有挑战性。在此,制备了用于过氧一硫酸盐 (PMS) 活化的单个 Co 原子掺杂 g-C3N4,以揭示高价钴氧基物质 [Co(IV) = O] 与单线态氧 (1O2) 的界面反应,并构建一个节能的 Fenton 样系统。实验结果表明,该系统可以用 0.355 min-1 的 kobs 实现 99.6% 的卡马西平去除,而 PMS 的分解是温和的(0.0988 和 0.00917 min-1 分别在 0-2 和 2-30 分钟内)。机理研究揭示了 Co(IV) = O 对 1O2 的反应,这调节了 Co 位点的减少,从而减慢了 PMS 的分解速率。但 1O2 和 Co(IV) = O 的协同作用维持了系统的高活性。结合第一性原理计算,PMS 的最佳演变路径为 PMS → OH*→Co(IV) = O → OO*→1O2,伴随着 Co(II)/Co(III)/Co(IV) 氧化还原循环。该工作提出了一种新的界面反应,即 Co(IV) = O 触发 1O2 生成,从而构建了一种节能的 Fenton 样系统,并为开发高效、低消耗的净水技术提供了新思路。
更新日期:2023-06-30
中文翻译:

高价钴-氧代物质触发单线态氧,在单个 Co 原子掺杂 g-C3N4 中触发单线态氧快速降解污染物以及温和的过氧一硫酸盐分解
为高效 Fenton 类过程定制界面反应途径和低消耗策略是环境可取的,但仍然具有挑战性。在此,制备了用于过氧一硫酸盐 (PMS) 活化的单个 Co 原子掺杂 g-C3N4,以揭示高价钴氧基物质 [Co(IV) = O] 与单线态氧 (1O2) 的界面反应,并构建一个节能的 Fenton 样系统。实验结果表明,该系统可以用 0.355 min-1 的 kobs 实现 99.6% 的卡马西平去除,而 PMS 的分解是温和的(0.0988 和 0.00917 min-1 分别在 0-2 和 2-30 分钟内)。机理研究揭示了 Co(IV) = O 对 1O2 的反应,这调节了 Co 位点的减少,从而减慢了 PMS 的分解速率。但 1O2 和 Co(IV) = O 的协同作用维持了系统的高活性。结合第一性原理计算,PMS 的最佳演变路径为 PMS → OH*→Co(IV) = O → OO*→1O2,伴随着 Co(II)/Co(III)/Co(IV) 氧化还原循环。该工作提出了一种新的界面反应,即 Co(IV) = O 触发 1O2 生成,从而构建了一种节能的 Fenton 样系统,并为开发高效、低消耗的净水技术提供了新思路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号